Protoreaction of Protoplasm
Vladimir V. Matveev
Laboratory of Cell Physiology, Institute of Cytology,
Russian Academy of Sciences,
Tikhoretsky Ave
Full text [PDF]
Abstract. My goal is to describe briefly the universal cellular
reaction (UCR) to external actions and agents. This general reaction was the
main subject of investigation by the scientific school of the outstanding
Russian cytologist, Dmitrii Nasonov (1895-1957). The UCR consists of two phases
of complex changes in cellular viscosity and turbidity, in the cell's ability to
bind vital dyes, in the resting membrane potential, and in cellular resistance
to harmful actions. Works from the Nasonov School have shown that these changes
are based on structural-functional transformations of many cell proteins that
react uniformly to actions of different physical and chemical nature. In
general, these complex changes do not depend on cell type, indicating the
universal and ancient nature of the UCR as well as its general biological
significance. A new interpretation of the mechanism of the universal reaction is
proposed in this paper, and a possible role for contractile proteins in the
mechanism of the UCR of muscle cells is presented. In addition, the concept
of cell hydrophobicity is introduced. Nasonov's School proposed a concept
of physiological standardization that allows comparison of data obtained by
different investigators and that will also be described here.
Introduction. According to an old Indian parable, well known in
Russia, residents of the city of blind people asked several respected citizens
to act as experts and to describe to them the nature of an elephant, about which
they had heard much. It happened that one of these animals was present near the
walls of their city. One expert who examined the elephant’s leg by feeling it
came to the conclusion that the elephant was a column. Another expert, upon
touching carefully the animal’s tail, stated that the elephant was a rope. The
expert who got the tusk was absolutely sure that the elephant resembled a
ploughshare. Clearly, the experts failed to agree and continued to dispute all
their lives, since each one felt that their case was based firmly on established
facts. Thus, each of them was in the right, but all of them were wrong on the
whole.
Cell physiology and the scientists dealing with study of this discipline
somewhat remind us of the meaning of this parable. To some of them, cell
physiology focuses on the plasma membrane, to others the nucleus is the key, yet
others prefer seeing the key to the mysteries to be found in signaling pathways.
The “touching” of individual cell parts continues in contemporary cell biology.
Fortunately, the cell itself gives us examples of its reactions that imply the
basis for generalizations, for a broad view of cell physiology. One such example
is the universal cellular reaction (UCR) to external actions, which was studied
in detail by the physiological school of the outstanding Russian scientist,
Dmitrii Nasonov (1895-1957), founder of the Institute of Cytology of the Russian
Academy of Sciences, and author of 117 publications including two monographs. At
present, the total number of publications from the Nasonov School is estimated
to be between 400 and 500. It is true that Nasonov himself called this reaction
unspecific, rather than universal. But I consider the term “universal” to be
more accurate and to better reflect the physiological and biological
significance of this reaction, and I will apply this terminology here. The UCR
is the uniform complex of substantial changes, apparently occurring in all cell
types, in response to external actions of all kinds. The goal of this article is
to describe some of these forgotten investigations, and to consider them in
terms of another paradigm, the Association-Induction Hypothesis (14, 15) that
seems to me to be a suitable basis for such an analysis. The necessity to
reinterpret the results of the Nasonov’s School and its heritage seems
reasonable because the corresponding literature, already old, can be found to
contain only the phenomenological or quite general accounts of
the UCR. However, it seems to me that something better can be suggested in terms
of contemporary biology. I hope the reader will agree that, in the framework of
this brief paper, only a schema of this new approach to the problem can be
presented. I will consider this task completed if I manage to present to the
reader at least the general notion of the universal cellular reaction, and of
its possible mechanism.
A universal reaction of the living cell. One of the least
understood properties of the living cell, apparently outside the scope of modern
science, is its ability to respond to stimuli of different natures by
the same standard complex of structural and functional responses. It is
upon this phenomenon that the main efforts of Nasonov’s School were focused. In
these studies major attention was devoted to changes in cell properties, rather
than to descriptions of its steady states. A simple but quite efficient method
to investigate cell changes was to study binding of vital (non-toxic) dyes by
cells. This procedure became the key approach in studies by the School and was
also accompanied by studies of such physical characteristics as turbidity
(transparency) of cytoplasm and nucleoplasm, their viscosity, biopotentials, and
resistance to damaging actions by the agents discussed below.
The list of actions on the cell that were studied included: increased
temperature, mechanical stress, hydrostatic pressure, electric current, general
anesthetics, pH, medium tonicity, salts of heavy metals, hypoxia, and sound
irradiation (200-7000 Hz, 94 dB). These studies used epithelial, nerve, muscle,
connective tissue, the germ cells of various worms, echinoderms, coelenterates,
molluscs, crustaceans, insects, and other invertebrates, as well as
representatives of protozoa and some plant cells (see 20 for references).
Based on these abundant data, I present in Fig. 1 a universal complex of
cellular changes in response to the agents named above. It includes changes of
cell properties in the first phase and then in the second phase of both types of
responses.
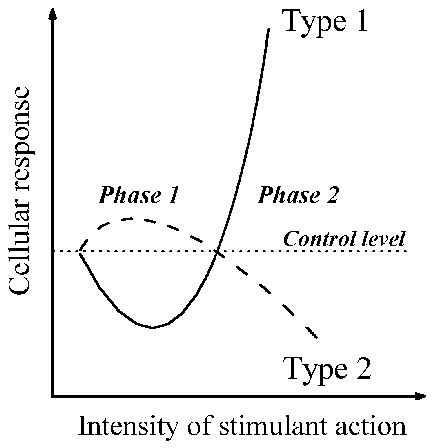
Fig. 1. Schematic presentation of the synchronous changes in cells that develop
during the course of the universal cell reaction, in response to actions of
various kinds. Changes in the cell’s turbidity and viscosity, and of its ability
to bind vital dyes, occur as described by type 1. Changes in cell resistance to
harmful agents, and of the resting membrane potential, occur as in type 2.
Further details are given in the text.
Changes of the first type. Many works have
established that changes in turbidity of the cytoplasm and nucleus always occur
in response to various actions on the cell. The second phase of the reaction is
easily observed under the microscope: first the entire cell starts fluorescing
with a pale blue light, then white structures appear, and turbidity increases.
These changes are especially evident in nuclei, in which they appear even
earlier than in cytoplasm. During the first phase of the reaction, the
transparency of the protoplasm increases and, being a visual response, is best
recorded by instrumental methods. In this paper the term “protoplasm” will be
used, as it was in the days of Nasonov, to refer to the entire living substance
of cells. On the whole, these changes can be characterized as follows: the size
of intracellular colloids initially decreases, and later, at the second phase of
the reaction, begins to increase, seemingly due to aggregation of the cytomatrix.
Another typical change characterizing the UCR response is an increase in
intracellular viscosity. Not infrequently, it becomes possible to record a
decrease of the viscosity (the first phase of type 1) before the beginning of
its increase (the second phase).
Nasonov’s School studied in the greatest detail the ability of cells to bind
vital dyes. At rest, the cell is almost never stained with vital dyes, and this
is especially true for the nucleus. However, under certain actions, the nucleus
and cytoplasm start adsorbing the dye intensively, and then dye adsorption
increases many times (up to 500% of the control or resting level, see Fig.1).
Especially intensively stained are the structures that are found in the nucleus,
such as chromatin granules, nucleolus and nuclear envelope. In contrast it was
found that during Phase 1 the ability of cells to bind dyes decreased by 10-30%.
In both cases, the % values refer to the degree of dye binding by all of the
cells in the population studied.
Changes of the second type. Early in Nasonov’s
career, great interest was given to the data involved with the first phase of
type 2 of the universal reaction – namely, the increase in resistance of cells
damaged by heat or chemicals. This increase in resistance and stability was
manifested, in particular, by an increase in the ability of isolated muscle to
survive in Ringer’s solution. Such stabilization of muscle and other cells was
observed under the action of D2O, general anesthetics and a variety of sugars,
salts, vital dyes, and other compounds at concentrations at which development of
the UCR was delayed at the first phase. At a higher doses (concentrations) the
increase in resistance is replaced by its decrease during development of the
second phase of the UCR. In that case, the cells become much more sensitive to
damaging agents (see 28 for references).
Study by the School on the cellular resting membrane potential, recorded by
extra- and intracellular methods showed that membrane hyperpolarization
(relative to the resting state) took place during the early stages of
development of the reaction. Later, after a longer or more intensive action
(i.e. at the second phase) depolarization then begins (see 28 for references).
Such results can be added to other characteristics of the UCR. For example,
during the second phase an acidification of the nucleus and cytoplasm occurs, as
well as the release from the cell of various substances including K+
along with the simultaneous influx of Na+ and Cl- (20).
It should be noted that the first phase of the UCR is less intense and of
shorter duration than the second phase, therefore, its recording requires a high
precision experiment.
A matter of principle importance should be especially emphasized: the
universal reaction can develop not only in the cell as a whole, but also in its
individual parts, depending on the nature of the action. Hence, the UCR can
also be a localized process. This peculiarity fascinated Nasonov and
was always at the center of his attention; he believed that there was no
principal difference between the localized reaction and the reaction of the
whole cell in terms of the spreading excitation of the action potential (20).
Finally, after cessation of a given action on the cell, all subsequent changes
show a reversed pattern and the cell gradually returns to the resting state. In
particular, dyes are released by the cell into the surrounding solution against
their concentration gradients during recovery. The cytoplasm and nucleus revert
to being colorless, and K+, various phosphates and other substances
that left the cell are now taken up once more.
These changes can be summarized as follows: the first phase of the UCR is
characterized by an increase in cell stability, an elevated resting membrane
potential, and a decrease in cellular viscosity and turbidity, as well as a
slight decrease in the ability of the cell to bind vital dyes.
The second phase is characterized by a decrease of cell stability and resting
potential, a rise of viscosity and turbidity of the protoplasm, and a
significant increase in the ability of the cytoplasm and nucleus to bind vital
dyes.
Why protoreaction? Experimental information accumulated over 40
years of investigations allowed Nasonov to conclude that his universal cellular
reaction is based on reversible changes of cellular proteins (20). Indeed,
changes in protein solutions in vitro are qualitatively similar to
changes observed in living cells under comparable conditions. Thus, proteins
lose solubility and aggregate, often with a rise in the viscosity of their
solutions, and their ability to bind dyes increases when stressed. On the other
hand, the actions that increase cell resistance also increase the stability of
isolated proteins. Thus, agents such as ethanol and chloral hydrate at a
concentration at which they increase resistance of the frog sartorius
muscle also increase stability of the glycerinated sartorius muscle
models (29), as well as of isolated actomyosin (16, 17). Those are important and
possibly profound observations.
The above changes in protein solutions are as universal as the UCR and they are
induced by the actions of practically any physical or chemical agent. The
opposite is also true: thus, agents able to produce these changes in proteins
in vitro also elicit the UCR (20). Comparing these many observations,
the conclusion was easily reached that even the very first proteinoids (6) in
evolution had the capability to produce the universal reaction, and that has
general biological significance (20). It is in this context that I have referred
to the universal reaction as the “protoreaction”, as it is this
response that must be the basis through evolution for the formation of numerous
regulatory systems in cells and, to a degree, will continue to be reflected in
physiological reactions in contemporary cells. But this term also has another
meaning: in the protoreaction, we should find the fundamental processes that
must be responsible for the physical basis of life. So what is this physical
basis?
Physiological atom of the living cell. Experts who consider
that cell physiology is very heavily influenced by membrane biology will hardly
set about explaining the mechanism of protoreaction, since we have already
stated that it takes place not only in whole cells, but also in local
intracellular areas as well as in membrane-deprived structures such as
glycerinated cell models and isolated proteins. For this reason, a promising
basis for analysis of protoreaction is, in my opinion,
Ling’s
Association-Induction Hypothesis (AIH) that has been developed by its author for
4 decades and strives to be revolutionary, a break-through in viewpoints on the
cell based on its bulk-phase system (15, 19).
According to Ling’s
theory, the physical basis for life is an ion-water-protein complex – the
smallest structural unit that has the capability for protoreaction:
K+-H2O-PROTEINunf-ATP <–––> PROTEINf
+ H2O + ADP + Pi + K+,
where PROTEINunf represents unfolded protein molecules, whose
polypeptide chains are accessible to the solvent water; where K+-, H2O-,
ATP – represent protein-bound potassium ions, water, and ATP; and PROTEINf
– the folded protein molecule, in which a significant part of the polypeptide
chain becomes inaccessible to water (see Fig. 44 in reference 15 for further
details).
The left part of this equation refers to a cell in the resting state,
and the right part to the state of activity or excitation. According to the AIH,
it is such local changes that occur during action potentials, muscle
contractions, and other forms of cellular activity. Transitions from the resting
to the active are accompanied by the release of free energy necessary to perform
biological work (15).
Transitions between these two states of the ion-water-protein complex represent,
basically, a sol-gel transition or a cooperative phase transition. According to
this view, the triggering switches between these phases are what generates the
dynamics of life. These transitions are based on regulated conformational
protein changes that are not simply related to shifts of atoms. It is probably
more useful to evaluate relative conformational changes by their accompanying
thermodynamic changes rather than by values of mechanistic shifts of parts of
the molecules. If that approach is taken, the ion-water-protein complexes and
their protoreaction are in essence the physiological “atoms” of the cell in the
sense that this is the minimal structural entity able to produce the
main interactions responsible for cellular life, and its response to external
disturbance. I suggest that the living cell acts as if it is composed of such
“atoms”, the various combinations of which are then included into organelles,
the cytomatrix and various other cell structures. These “atoms” can acquire
features of specialization, but the main structural-functional principles of
their activity remain unchanged, so I will consider these “atoms” to be the
basic units of the living cell. Finally, I should note that not only the whole
protein molecule can act as a basic unit, but also that parts of it can operate
that way. In addition, when associations of such “atoms” take place with a high
degree of cooperativity, these “associations” or “complexes” can be regarded, in
some cases, as one “atom.”
This is the AIH logic, as I understand it. It seems to me that, on the whole,
shifts of dynamic equilibrium between two states of the basic unit reproduce, at
the elementary level, the protoreaction of cells, as shown in Fig. 1. I
suggest that these dynamics are the two states of a binary code, upon which cell
physiology operates.
Among other things I will later use the studies on dye adsorption done by the
Nasonov School to illustrate the basic features of the protoreaction. But first
it is necessary to examine a question not usually considered in this fashion:
what is the nature of cell hydrophobicity?
Cell hydrophobicity: a missed role for proteins. For a long
time, and up to the present, the term hydrophobicity was mostly has been
associated chiefly with lipids. The well-known Meyer-Overton rule was always a
strong argument in favor of the lipid nature of biomembranes and of the membrane
theory of anesthesia. Until the 1960s, to be “hydrophobic” was synonymous with
being “lipid”, and the hydrophobic properties of the cell were explained by the
presence of its lipid membranes, first of all, and primarily the plasma
membrane. Indeed, based on these concepts, numerous “lipid” theories of
anesthesia were put forward.
However, in the 1960s, when studying thermodynamic characteristics of the
thermodynamics of protein folding and unfolding, Brandts (3) was the first to
prove convincingly that during the folding of a protein molecule, hydrophobic
areas are formed internally which are inaccessible to water. Initially the
thermodynamics of conformational transitions in proteins was the subject of
study by a small group of specialists. However, with time, it has become evident
that hydrophobic areas within cells are represented not only by lipids, as this
was thought for more than 70 years, but also by proteins. The importance of this
reappraisal is emphasized by the fact that, after water, protein is the most
abundant of all other constituents, comprising up to 65% of the dry mass of
cells, and greatly exceeds the total amount of lipid. What I propose here is
that the volume of the hydrophobic protein phase can greatly exceed that of the
hydrophobic lipid phase. However, I also recognize that the full significance of
this observation has not been understood and seemingly not accepted by
contemporary cell physiologists in terms of paradigms and working hypotheses.
The next development essential in our understanding of cell hydrophobicity came
from the works of Katz and Simon (11) and Halsey et al. (9), who came to the
principally important conclusion that there was no difference between
the physical properties of hydrophobic sites of lipids and proteins as revealed
by a thorough thermodynamic analysis. In other words, hydrophobic compounds
within cells will interact with any other hydrophobic site, regardless
of location be it in proteins or in lipids. This statement has an important
consequence that will become clear when we consider the example of valinomycin,
a selective potassium ionophore. It is accepted as axiomatic that this
rather hydrophobic compound is dissolved only in the lipid phase of the cell’s
plasma membrane, and becomes a K+ carrier by virtue of its
concentration gradient. As a result and as repeatedly observed, cells treated
with valinomycin loses K+. This “dogma” first appeared over 50 years
ago when nothing was known about the hydrophobic phase(s) in proteins, and still
persists to this day (10, 25). But we also know now that such overly simplistic
interpretations of valinomycin’s effect on the cells are quite unacceptable. At
present, it is evident that valinomycin can be inserted into any
hydrophobic phase, regardless of its nature, be that lipid or protein. Hence,
valinomycin can essentially change properties not only of membranes, but also of
proteins (including those of the cytomatrix); therefore, it is no longer correct
to explain the mechanism of action of this compound on the cells only
by the action on changes in the permeability of the plasma membrane.
Interestingly, this statement, made on the basis of general considerations, has
become now been confirmed experimentally. It turned out initially that
valinomycin also had peculiar “side effects”. Thus, it was revealed that
valinomycin had the ability to interact directly with cytochrome c oxidase (21,
26, 27), Ca2+-ATPase (2), and (Ca2+,Mg2+)-ATPase
of skeletal muscle sarcoplasmic reticulum (5). It seems reasonable to suppose
that other even partially hydrophobic ionophores might also directly interact
with proteins. That topic seems worthy of further careful study.
Thus, after decades, it seems that the Meyer-Overton rule is neither a proof of
the lipid nature of membranes, nor evidence for the key role of membrane lipids
in anesthesiology. This rule merely indicates a role for hydrophobic
interactions in the cell permeability to the so-called lipophilic compounds.
The term “hydrophobic interaction” often is considered to be synonymous with
non-specificity. In reality, that term of hydrophobic interactions is as
non-informative about the degree of their specificity as is the use of such
terms as hydrogen bonds or ionic interactions. All these terms merely indicate
the physical nature of the interaction, rather than indicate any degree of the
level of their specificity. The latter quality depends on numerous additional
factors that are realized in the microenvironments of the interacting molecules.
At present, the protein theory of anesthesia is commonly accepted, according to
which the targets of the anesthetic effect are hydrophobic sites located in
proteins (7), and this is of principal importance for the issues I consider in
this paper.
Phase transitions of basic units and cell hydrophobicity. The
evidence at my disposal suggests that the basic unit protoreaction, apart from
other changes, leads to the appearance in the cell of a new physico-chemical
factor – hydrophobic areas formed by proteins. This statement is based on
postulated properties of the basic units, according to which a shift of the
dynamic equilibrium between two states of the basic unit (unfolded <–––> folded)
to the right will bring about a relative increase in the number of protein
molecules in the folded state. This will favor the formation of protein
hydrophobic sites (areas, domains, pockets) by virtue of the participation of
hydrophobic side groups, both inside the protein molecule and in intermolecular
contacts (3). Thus, Ling’s
model predicts that at transition of the protoreaction into the second phase of
its development (see Fig. 1), the volume of the cellular protein hydrophobic
phase will increase. However, it is to be stressed that
Ling does not consider
such a possibility in his extensive writings (14, 15).
An increase of the hydrophobic phase volume fundamentally changes the conditions
of the intracellular environment and inevitably leads to a massive
redistribution of all lipophilic compounds within the cell and between
the cell and the external medium. Such a redistribution should also involve key
substances such as ATP, since this compound is distinguished by significant
hydrophobicity (13). That seems to be a rather significant point with regard to
the UCR.
During Nasonov’s time, information on the properties of proteins was scarce. It
was cautiously believed by his School that development of the protoreaction
leads to the appearance of additional fixed charges on proteins in cells, with
which vital dyes, known to be organic ions, presumably interacted. However, in
the review by Leo et al. (13) it is pointed out that all vital dyes are
characterized by high lipophilicity, whereas the charge on these compounds
produces no essential effect on their hydrophobic interactions with other
substances. This result is particularly true for organic cations (24).
One of these organic cations, the vital dye neutral red, was widely studied by
the Nasonov School, and its use allowed them to obtain most of the data on an
increase of dye binding by the cell during the second phase of the protoreaction.
Of great importance in this connection, is the fact that neutral red is no
different from general anesthetics (17) as far as its mechanism of interaction
with cell structures is concerned: both the dye and general anesthetics interact
with cell hydrophobic sites. Thus, vital dyes are, in essence, indicators of
the volume of the cell hydrophobic phase formed by intracellular proteins.
Nasonov explained the increase in dye binding in the course of the protoreaction
as being due to the “initial stage of protein denaturation”, since proteins
denaturated in vitro also bind dyes better than their native
conformations. Both in Nasonov’s works and in the context of the present paper,
use the term “denaturation” (i.e. loss of natural properties) seems
inappropriate, as it implies irreversible and probably lethal changes. In
discussions between Nasonov and his opponents, it was argued that the cell is
able to repair “denatured” proteins, and specifically those with conformational
modifications similar to the denaturated state. However, from the point
of view of the above-considered dynamics of the basic unit states, restoration
of the cell to its initial state after protoreaction looks not so much like
reparation, but more like the normal change of the basic unit states
involved in mechanism of UCR. Inappropriateness of the term “denaturation” was
also indicated by numerous data obtained by Nasonov and his colleagues,
according to which the normal functional activity of cells (secretion, muscle
contraction, nerve impulse propagation, transmission of synaptosome signals,
etc.) is also accompanied by an increase in the cellular viscosity, turbidity
and dye binding (see 20 for references).
Of great interest is the question of how vital dyes leave cells,
against their concentration gradients, after completion of the protoreaction and
a return of the cells to their resting state. First, it could be because a
transition to the resting state is accompanied by a decrease in the volume of
the hydrophobic phase (i.e. a decrease in the number of the dye-binding
hydrophobic centers). Second, according to the AIH, a large fraction of cell
water in the resting condition is in a state of restricted mobility (“bound”)
and is a poor solvent for large ions and various molecules (15). As a result,
these are excluded from intracellular water into the surrounding solution. On
the other hand, if we interpret the data according to the membrane theory, it
becomes necessary to postulate the existence of active transport systems for
each of the dyes studied by Nasonov’s School.
The concept of the basic unit helps explain as well the first phase of
protoreaction when the ability of a cell to adsorb dyes is slightly reduced. The
general explanation is based on the assumption that the cell contains a small
number of basic units in a folded state under resting conditions since the
balance “unfolded units <---> folded units” is dynamic. If some influence on a
cell leads to an even greater displacement of the dynamic balance to the left,
the total volume of a protein hydrophobic phases in a cell will decrease in
comparison with the resting state. As a result, the cell’s ability to bind
lipophilic dyes will also decrease. For example, in the case of the action of
general anesthetics interacting hydrophobically with cellular proteins,
thermodynamic factors could play an important role. For example, at a certain
anesthetic concentration it could be advantageous thermodynamically for protein
hydrophobic side groups to make contact with the mixed solvent (water +
anesthetic) instead of with each other (3). As a result, folded conformations of
basic units, available in the resting state of a cell, could become unstable and
unfold, and expose its hydrophobic groups to the mixed solvent. In that fashion,
the dynamic balance between the two states of the basic unit will be shifted to
the left to a greater degree than in the resting condition.
Another important factor in these processes is an increase of cellular ATP
during the first phase of the protoreaction (see 28 for references). According
to the AIH, an increase in cellular ATP concentration should lead to a shift to
the left of the equilibrium between the two states of the basic unit.
Ling (15) believes that
ATP is the “cardinal adsorbent” and a key component of the AIH. In the context
of my paper an increase in ATP concentration would strongly affect the dynamic
equilibrium between the two states of the basic unit: an increase in ATP
concentration would shift the equilibrium to the left, while a decrease would
shift the equilibrium to the right.
The significance of the increase in hydrophobicity of the cytoplasm and nucleus
for the functions of the cytoskeleton, signaling pathways, genome, and other
important cellular mechanisms remains virtually unknown and has yet to be
investigated.
Intracellular viscosity. I should first note that the studies
done by the Nasonov School involved descriptions of macroviscosity due to
limitations in the methodology of his era. Changes in the cytoskeleton are the
first that come to mind as an explanation for the changes in viscosity during
the course of the protoreaction. However, years of study on the effects of
anesthetics on cytoskeletal elements have shown that these compounds
depolymerize microtubules and microfilaments at clinical concentrations (1).
Thus, at the phase of cellular narcosis (i.e. at the second phase of the
protoreaction) when the viscosity increases, this is opposite to what would be
expected from disassembly of the cytoskeleton.
Taking into account the basic unit properties, another explanation could involve
the bound state of intracellular K+. During tetanic contraction of
muscle and ethanol exposure, under conditions when the muscle cell protoreaction
reaches the second phase of its development, K+ is known to leave the
muscle due to K+ desorption from the K+-binding matrix
(30). K+ efflux from muscle during excitation is a well-known. In the
AIH context, free anionic groups on proteins produced by K+
desorption interact with fixed cationic groups on the same protein, or adjacent
ones. As a result of these interactions of fixed ions, there appears a
three-dimensional network of protein molecules bound to each other in the cell,
or in localized parts of it. This network is believed to increase the viscosity
significantly. A role in the stabilization of such a network can also be played
by interprotein hydrophobic interactions, where hydrophobic side groups of
adjacent protein molecules interact with each other, thereby contributing to the
stabilization of protein complexes or aggregates. Taking into account the high
protein concentration in cells, this “polymerization” of basic units can proceed
very fast, and involve large parts of cells or even their entire volume. Such
aggregations will inevitably lead to an elevation of viscosity, an increase in
the sizes of intracellular particles, and, hence, to an increase of cell
turbidity. Taking all this into account, the cytoskeleton does not seem to play
the key role in mechanisms underlying the increase in viscosity.
Recall that, during the first phase of protoreaction, the viscosity and
turbidity fall below their resting levels. One can account for those
observations by a process involving the absorption. To do so, extra anionic
groups fixed to the basic unit are needed, some of which come from sites that
were previously occupied by other fixed charges during the resting state.
According to AIH logic, the number of fixed anionic groups available for K+
binding increases when the cellular ATP concentration rises. This theoretical
prediction is in accord with the above-mentioned data showing an increase in ATP
during the first phase of the protoreaction (see 28 for references). Thus, an
increase in ATP synthesis and its excessive binding (compared with the resting
state) by basic units results in the breakage of an additional number of ionic
bonds between proteins, and an increase in the number of fixed anionic
groups that can bind K+. It is further proposed that the above is
accompanied by a partial “depolymerization” of the three-dimensional network of
protein molecules, because some of the ionic bonds participating in its
stabilization are broken. Such a process of weakening of interprotein
interactions would also be reflected as a decrease in cell viscosity and an
increase in its transparency as a result of the dissociation of protein
aggregates.
Unfortunately, cell viscosity and K+ content, as far as I know, have
always been studied separately. Consequently, one can only refer to indirect
evidence in favor of the above-described mechanism. Such indirect evidence comes
from an interesting work by Troshina (31) showing that, under the action of
insulin on frog sartorius muscle, the resting potential of the muscle
fibers increases, while their ability to adsorb neutral red decreases; hence,
insulin produces the first phase of the protoreaction in this muscle, during
which viscosity and turbidity of the sarcoplasm are known to decrease. On the
other hand, it is well established that insulin increases the K+
content in muscle (4) which, according to the AIH, could be due to the
appearance of additional sites for K+ binding, and to a corresponding
decrease in stability of the protein matrix, as discussed above. As a result,
the dynamic equilibrium in the basic unit shifts to the left to a greater degree
than in the resting state, leading to a decrease in viscosity and the ability to
bind vital dyes.
It seems that the same effect can be produced by any action that increases
cellular ATP content since this increase is accompanied by a rise in
intracellular K+ content (8). In this connection, it is interesting
that these actions (classical for Nasonov’s School) lead to an increase in
creatine phosphate and ATP in the cell, since these also increase cell
resistance / stability (see 28 for references). Based on the above discussion,
the following “rule” can be formulated: the greater the shift of dynamic
equilibrium between two states of basic units toward the left, the higher the
cell resistance and stability.
Thus, major cause of changes in colloidal properties of cells, including
rheological ones, seems to be assigned to the state of K+-binding by
the cellular matrix, the extent of which differs at different phases of the
protoreaction.
Limiting proteins. From the point of view of the AIH, basic
units play the key role in maintaining fundamental physico-chemical conditions
of the intracellular medium, which underlie the entire structural-functional
organization of cells. This gives good grounds to the belief that the loss by
basic units of the ability to perform their function would be sufficient for
cell death. If so, the proteins that are the structural basis of these units can
be called “limiting proteins” – those that play a critically important role in
providing the necessary conditions for metabolism and, therefore, life.
This theoretical anticipation has been confirmed experimentally. Rosenberg et
al. (23) studied the kinetic parameters of thermal protein denaturation and
thermal death of unicellular and multicellular organisms. They came to the
paradoxical conclusion that denaturation of one protein, or of a small number of
proteins with close properties (that the authors called limiting proteins)
were sufficient for thermal death of a cell or organism. From the point of view
of the AIH, such proteins might be those of the K+-binding cell
matrix. An important question arises: what can be said about the nature of these
proteins?
As already noted here, the first protoreaction phase is characterized by an
increase in cell resistance to damaging factors, including thermal damage. For
instance, in Ringer’s solution containing 6 mM chloral hydrate or 680 mM ethanol
the survival time of frog sartorius muscle is twice as long as that of control
preparations. Similar effects have also been obtained using other chemical
agents (see 28 for references). The question then is: which intracellular
structures and/or proteins are the targets of the actions responsible for an
increase in resistance of the muscle cell? Of course, there are many proteins in
cells, and their properties differ greatly. For instance, the maximal
stabilizing effect of ethanol on ribonuclease is achieved at 2000 mM ethanol
(3), whereas 680 mM is sufficient in the case of actomyosin (16).
In this connection, it is interesting to compare data obtained on living
muscle and glycerol-treated muscle models (see 28 for references),
and on isolated actomyosin (12, 16, 17). It has been established that
stability of all these preparations increased over the same concentration
range for chloral hydrate (maximum effect at 6 mM) and ethanol (maximum
effect at 680 mM). In other words, this response of the living cell is, to some
degree, reproduced by isolated proteins, specifically, by the
contractile muscle proteins. This astonishing observation merits more detailed
study.
But why does actomyosin give such a good correlation with living muscle in terms
of these effects? Is this because of the high content of these proteins in
muscle? Or do the contractile proteins play some additional key role in enabling
viability of muscle cells? One possible answer might be connected to the fact
that the contractile proteins bind the majority of K+ present in
muscle (see 15 for references) and thereby are the structural origins of the
basic units of the UCR in muscle cells. If this is really so, then the
contractile proteins represent the K+-binding matrix, whose stability
is entirely responsible for cell viability. In that case, it is clear that
inactivation of the K+-binding matrix alone could make functioning of
muscle cells impossible. And, to the contrary, actions that stabilize
contractile proteins in vivo also make the treated muscle cell more
resistant to malfunction. Apart from the key role of contractile proteins as the
basic units, they also play an important role in the transmission of signals
within muscle cells (18). Under such circumstances, and in this context,
contractile proteins can indeed be considered limiting, and the above-mentioned
experimental data provide additional evidence in support of the conclusions of
Rosenberg et al. (23) about the cause of the thermal death of cells and
multicellular organisms.
Protoreaction as a physiological standard. It is easy to see
that the protoreaction represents a non-linear response of cells to some action.
This means that the same stimulus can produce different results depending on its
intensity. This partly explains numerous controversies in the literature, as
authors studying some particular property of the cell do not suspect that under
their experimental conditions, the protoreaction can develop, so that the cell
properties being studied depend essentially on the phase involved. Analysis of
the results obtained, without considering the physiological background under
which they are obtained, is not likely to be correct. So it is important to know
in which state of protoreaction cells are when they are being studied. Indeed,
it is very likely that protoreaction takes place in every case if a cell is
affected by any method. One can only compare those effects that are developed
against a background of the same phase of protoreaction (see Fig. 1)
according to the rule "all other conditions should be equal" (ceteris paribus).
In this way, numerous cell effects could be standardized, depending on the
protoreaction phase in which they were observed.
In my opinion, the best indicator of the protoreaction is a change in the
hydrophobicity of cells or of certain intracellular structures. Thus, an
increase in nuclear hydrophobicity might initiate some reactions, while
preventing others. For example, it is very unlikely that signalling systems in
cells will operate similarly in the hydrophilic (Phase 1) and hydrophobic (Phase
2) regions of cytoplasm or nucleus. All these issues are extremely interesting
and important to increase of effectiveness of science, but are almost entirely
uninvestigated.
Why can the protoreaction be used as a standard? Because the entire body of
scientific evidence accumulated by Nasonov’s School supports the claim, with
some degree of certainty, that the protoreaction is the only cell reaction
that, in spite of its complexity, has a universal and general biological
character. Furthermore, the complex changes occurring during development of the
protoreaction appear in all cell types, at the scale of the entire cell as well
as intracellular structures, including molecular complexes. The
structural-functional principles that underlie the protoreaction can be revealed
in greatly different ways in the nucleus, cytoplasm, organelles, during muscle
contraction, nerve impulse propagation, apoptosis, and so on, but the principles
themselves remain invariant.
Conclusion. Currently, the ideas, approaches and methods of
study developed by Nasonov’s School have essentially been forgotten. But it is
absolutely clear to those who still remember this page of history of Russian
science that the School studied some fundamental cell properties, whose
significance for biology is not understood up to the present time. It is
necessary to continue these investigations of the Nasonov School, at the least
because Nature never disappoints those who study successively its fundamental
manifestations. In my view, one such manifestation is undoubtedly the
protoreaction.
Here I have outlined merely the general scheme of the UCR / protoreaction and
its possible interpretation based on the Association Induction Hypothesis of
Gilbert Ling. It is
certainly evident that many aspects of this approach need further study and
experimental confirmation. But something else also seems evident: only after
carefully comparing the findings of the Nasonov School with the main features of
the AIH, which I tried to do here, does it becomes clear as to which issues need
further study. Formation of a plan of investigation is one of the challenges of
a good theory.
Acknowlegement. I am indebted to James Clegg for critical
comments on this article. I also wish to thank Leonid Pevzner and Denys Wheatley
for their comments.
REFERENCES
1. Allison, A.C., The effects of inhalational anesthetics on proteins. In:
Molecular Mechanisms in General Anaesthesia, Halsey, M.J., Millar, R.A. and
Sutton, J.A. (eds), Churchill Livingstone, Edinburg, London, New York, 1974, pp.
164-181.
2. Beeler, T.J. and Gable, K.S., Phosphate, nitrendipine and valinomycin
increase the Ca2+/ATP coupling ratio of rat skeletal muscle
sarcoplasmic reticulum Ca2+-ATPase. Biochim. Biophys. Acta 1994,
1189: 189-194.
3. Brandts, J.F., Conformational transitions of proteins in water and in aqueous
mixtures. In: Structure and Stability of Biological Macromolecules, Timasheft,
S. N., and Fasman, G. D. (eds), Marcel Dekker, Inc., New York, 1969, pp.
213–290.
4. Creese, R., D’Silva, J.L. and Northover, J., Effect of insulin on sodium in
muscle. Nature 1958, 181: 1278.
5. Davidson, G.A. and Berman, M.C., Interaction of valinomycin and monovalent
cations with the Ca2+,Mg2+-ATPase of skeletal muscle
sarcoplasmic reticulum. J. Biol. Chem. 1985, 260: 7325-7329.
6. Fox, S.W., A theory of macromolecular and cellular origins. Nature 1965, 205:
328-340.
7. Franks, N.P. and Lieb, W.R.,
Seeing the light: protein
theories of general anesthesia. Anesthesiology 2004, 101: 235-237.
8. Gulati, J., Ochesenfeld, M.M. and Ling, G.N., Metabolic cooperative control
of electrolyte levels by adenosine triphosphate in the frog muscle. Biophys. J.
1971, 11: 973-980.
9. Halsey, M.J., Brown, F.F. and Richards, R.E., Perturbations of model protein
systems as a basis for central and peripheral mechanisms of general anaesthesia.
In: Molecular interactions and activity in proteins. Porter, R. and Fitzsimons,
D.W. (eds), Excerpta Medica, Amsterdam, Oxford, New York, 1978, pp. 123-132.
10. Inai, Y., Yabuki, M., Kanno, T., Akiyama, J., Yasuda, T. and Utsumi, K.
Valinomycin induces apoptosis of ascites hepatoma cells (AH-130) in relation to
mitochondrial membrane potential. Cell Struct. Funct. 1997, 22: 555-563.
11. Katz, Y. and Simon, S.A.,
Physical parameters of the anesthetic site. Biochim. Biophys. Acta 1977,
471: 1-15.
12. Kovaleva, T.A., Suzdalskaya, I.P., Butiagina, N.V., Stepanova, T.P. and
Gnetov, A.V., Enhancing the temperature stability of muscle proteins under the
action of chloral hydrate and urea. Fiziol. Zh. SSSR Im. I. M. Sechenova 1991,
77: 159-162. (In Russian)
13. Leo, A., Hansch, C. and Elkins, D.,
Partition coefficients and their use.
Chem. Rev. 1971, 71: 525-616.
14. Ling, G.N.,
A Physical Theory of the Living State: the Association-Induction
Hypothesis, Blaisdell Publ. Co., Waltham, Mass., 1962.
15. Ling, G.N.,
Life at
the Cell and Below-Cell Level. The Hidden History of a Fundamental Revolution in
Biology, Pacific Press, New York, 2001.
16. Matveev, V.V., Denaturation time of actomyosin exposed to different
chemicals. Tsitologiia 1975, 17: 1278-1282. (In Russian)
17. Matveev, V.V., Enhanced stability of isolated muscles and actomyosin due to
action of anesthetics at subnarcotic concentrations. Tsitologiia 1987, 29:
197-201. (In Russian)
18. Matveev, V.V.,
Evidence of a new type of protein-protein interaction: desensitized actomyosin
blocks Ca2+-sensitivity of the natural one. A possible model for an
intracellular signalling system related to actin filaments. Physiol. Chem.
Phys. Med. NMR 2000, 32: 167-178.
19. Matveev, V.V.,
Revolution and counter revolution in cell physiology. Cell Biol. Int. 2002,
26: 305–308.
20. Nasonov, D.N.,
Local Reaction of Protoplasm and Gradual Excitation (English
Transl. by Halpern, Y.S.), National Science Foundation, available at Office of
Technical Services, US Department of Commerce, Washington, D.C., 1962.
21. Nicholls, P. and He, J., Direct and indirect effects of valinomycin upon
cytochrome c oxidase. Arch. Biochem. Biophys. 1993, 301: 305-310.
22. Porter, J., Pickup, R. and Edwards, C., Membrane hyperpolarisation by
valinomycin and its limitations for bacterial viability assessment using
rhodamine 123 and flow cytometry. FEMS Microbiol Lett. 1995, 132: 259-262.
23. Rosenberg, B., Kemeny, G., Switzer, R.C. and Hamilton, T.C.,
Quantitative evidence for protein denaturation as the cause of thermal death.
Nature 1971, 232: 471-473.
24. Roth, S, Seeman, P.,
The membrane concentrations of neutral and positive anesthetics (alcohols,
chlorpromazine, morphine) fit the Meyer-Overton rule of anesthesia; negative
narcotics do not. Biochim. Biophys. Acta. 1972, 255: 207-219.
25. Shapiro, H.M., Cell membrane potential analysis. Methods Cell Biol. 1994,
41: 121-133.
26. Steverding, D. and Kadenbach, B., Valinomycin binds stoichiometrically to
cytochrome c oxidase and changes its structure and function. Biochem. Biophys.
Res. Commun. 1989, 160: 1132-1139.
27. Steverding, D. and Kadenbach, B., The K+-ionophores nonactin and
valinomycin interact differently with the protein of reconstituted cytochrome c
oxidase. J. Bioenerg. Biomembr. 1990, 22: 197-205.
28. Suzdalskaya, I.P., Increase in stability of isolated tissues and proteins
caused by action of chemical stimulants. Tsitologiia 1977, 19: 839-849. (In
Russian)
29. Suzdalskaya, I.P., Kiro, M.B., Increase in the time of conservation of
contractility of glycerinated muscles exposed to subthreshhold concentrations of
chemical agents. Tsitologiia 1975, 17: 50-54. (In Russian)
30. Troshin, A.S.,
Problems of Cell Permeability, (English Transl. by Hell, M.G.
and Widdas, W.F.), Pergamon Press, London, 1966.
31. Troshina, V.P., Effect of insulin on natural properties of the frog muscle.
Tsitologiia 1964, 6: 751-754. (In Russian)
Full text [PDF]
ARTICLES CITING THIS ARTICLE
2011 Доценко О.И. и Мищенко В.О. Влияние низкочастотной вибрации на кислотную резистентность эритроцитов. Вiсник Днiпропетровського унiверситету. Бiологiя. Екологiя. Вип. 19, т.1. С. 22-30.
2011 Mentre P. Water in the orchestration of the cell machinery. Some misunderstandings: a short review. J Biol Phys, DOI 10.1007/s10867-011-9225-9.
2011 Kurakin A. The self-organizing fractal theory as a universal discovery method: the phenomenon of life. Theor Biol Med Model. 2011, 8:4.
2011 Digel I. Primary Thermosensory Events in Cells. Advances in Experimental Medicine and Biology, 704:451-468.
2011 Яхно Т.А. Агрегатное состояние и кооперативные реакции компонентов цельной крови в норме и патологии. Диссертация на соискание ученой степени доктора биологических наук. (Yakhno T.A. Aggregate state and cooperative reactions of whole blood components in health and disease. Doctoral dissertation, in Russian). [Автореферат] [Диссертация]
2010 Zhao QY. Protein Flexibility as a Biosignal. Critical reviews in eukaryotic gene expression, 20(2):157-170.
2008 Agutter PS. Elucidating the Mechanism(s) of Hormesis at the Cellular Level: The Universal Cell Response. American Journal of Pharmacology and Toxicology, 3(1):100-110.
2007 Agutter PS. Cell mechanics and stress: from molecular details to the 'universal cell reaction' and hormesis. Bioessays. 29(4): 324-33.
Web-Links
The journal "Physiological Chemistry and Physics and Medical NMR"
Pollack G.H. Water, Energy and Life. (Lecture) (Link 1) (Link 2)