Native aggregation
as a cause of origin of
temporary cellular structures
needed for all forms of cellular activity, signaling and transformations
Vladimir V. Matveev
Laboratory of Cell Physiology, Institute of Cytology,
Russian Academy of Sciences,
Tikhoretsky Ave
Full text [PDF]
Abstract. According to the hypothesis explored in this paper, native aggregation is genetically controlled (programmed) reversible aggregation that occurs when interacting proteins form new temporary structures through highly specific interactions. It is assumed that Anfinsen's dogma may be extended to protein aggregation: composition and amino acid sequence determine not only the secondary and tertiary structure of single protein, but also the structure of protein aggregates (associates). Cell function is considered as a transition between two states (two states model), the resting state and state of activity (this applies to the cell as a whole and to its individual structures). In the resting state, the key proteins are found in the following inactive forms: natively unfolded and globular. When the cell is activated, secondary structures appear in natively unfolded proteins (including unfolded regions in other proteins), and globular proteins begin to melt and their secondary structures become available for interaction with the secondary structures of other proteins. These temporary secondary structures provide a means for highly specific interactions between proteins. As a result, native aggregation creates temporary structures necessary for cell activity.
"One of the principal objects of theoretical research in any department of
knowledge
is to find the point of view from which the subject appears in its greatest
simplicity."
Josiah Willard Gibbs (1839-1903)
Introduction. To date, numerous mechanisms, signal pathways,
and different factors have been found in the cell. Researchers are naturally
eager to find commonalities in the mechanisms of cellular regulation. I would
like to propose a substantial approach to problems of cell physiology Ц the
structural ground that produces signals and underlies the diversity of cellular
mechanisms.
The methodological basis for the proposed hypothesis results from studies by the
scientific schools of Dmitrii Nasonov [1] and Gilbert Ling [2-6], which have
gained new appreciation over the last 20-30 years owing to advances in protein
physics [7] in the study of properties of globular proteins, their unfolding and
folding, as well as the discovery of novel states of the protein molecule: the
natively unfolded and the molten globule. The key statement for the rationale of
the present paper is that the specificity of interactions of polypeptide chains
with each other (at the intra- and inter-molecular levels) can be provided only
by their secondary structures, primarily
α-helices and
β-sheets.
NasonovТs school discovered and studied a fundamental phenomenon Ч the
nonspecific reaction of the cell to external actions [1], while works by Ling
[5] and his followers allow the mechanisms of this phenomenon to be understood.
The above-mentioned cell reaction has been called nonspecific because diverse
physical and chemical factors produce the same complex of structural changes in
the cell: an increase in the turbidity and macroscopic viscosity of the
cytoplasm and in the adsorption of hydrophobic substances by cytoplasmic
proteins. It is of primary importance that the same changes also occur in the
cell during its transition into the active state: muscle contraction, action
potential, enhancement of secretory activity (for details, see [8]). Hence, from
the point of view of structural changes, there is no fundamental difference
between the result of action on the cell of hydrostatic pressure and, for
instance, muscle contraction. In both cases, proteins are aggregated.
Nasonov called the cause of these changes the stages of cell protein
denaturation, as the changes of properties of isolated proteins during
denaturation are very similar to the changes in the cytoplasm during the
nonspecific reaction. As a result, the denaturational theory of cell excitation
and damage was created [1]. The structural changes of protein denaturation were
unclear in NasonovТs time. Nowadays, it is assumed that the denaturation is the
destruction of the tertiary and secondary structure of a protein. Below I give
two definitions, for the denaturation of natively folded (globular) proteins and
for natively unfolded proteins.
A key notion in physiology is the resting state of the cell. This is
implicit in the concept of the threshold character of the action of stimuli on
the cell, which has played a historical role in the development of physiological
science. It is the threshold that is the boundary between two states Ч rest and
activity. But in effect, all our knowledge about cells concerns active cells,
not cells in the resting state. It is in the active cell that variable changes
occur that can be recorded. Nothing happens in the resting cell, so there is
nothing to be recorded in it. Nevertheless, it is obvious that the resting state
is the initial cell state, the starting point for all changes occurring in the
cell.
What characterizes the structural aspect of the cell in the state of rest? It is
only in LingТs work [5] that I have found a clear answer to this question. The
answer can be interpreted as follows: if all resting cell proteins were arranged
in one line, it would turn out that most of the peptide bonds in this
superpolypeptide would be accessible to solvent (water), while only a few would
be included in secondary structures. When the cell is activated, the ratio
between the unfolded and folded areas is changed sharply to the opposite: the
proportion of peptide bonds accessible to solvent decreases markedly, whereas
the proportion included in secondary structures rises significantly. These two
extreme states of cell proteins, suggested by Ling, provide a basis for further
consideration.
If LingТs approach is combined with NasonovТs theory, we obtain several
interesting consequences. First of all, it is clear that proteins with maximally
unfolded structures form the structural basis of resting cells because they are
inactive, i.e., do not interact with other proteins or other macromolecules. The
situation changes when an action on the cell exceeds the threshold: completely
or partially unfolded key proteins begin to fold when new secondary protein
structures are formed. Owing to these new secondary structures, the proteins
become capable of reacting, i.e., intramolecular aggregation (folding of
individual polypeptides into globules) and intermolecular aggregation
(interaction of some proteins with others) begin. A distinguishing feature of
these aggregational processes is their absolutely specific character, which is
ensured by the amino acid composition, shape, and size of the secondary
structures. The structures appearing have physiological meaning, so such
aggregation is native and the secondary structures causing it are centers of
native aggregation. Another source of secondary structures necessary for native
aggregation is the molten globule.
The ability of cells to return to the initial state, the state of rest, means
that native aggregation is completely reversible, and the structures appearing
in the course of native aggregation are temporary and are disassembled as soon
as they cease to be necessary. Native aggregation can involve both the whole
cell and individual organelles, compartments, and structures, and activation of
proteins is of a threshold rather than a spontaneous character.
The meaning of the proposed hypothesis of native aggregation is that the primary
cause of any functional changes in cell is the appearance, as a result of native
aggregation, of temporary structures, continually appearing and disintegrating
during the life of the cell. Since native aggregation is initiated by external
stimuli or regulatory processes and the structures appearing have a temporary
character, these structures can be called signal structures.
Signal structures can have different properties: (i) they can be centers of
binding of ions, molecules (solutes), and proteins; (ii) they can have enzymatic
activity; (iii) they can form channels and intercellular contacts; (iv) they can
serve as matrices organizing the interactions of molecules in synthetic and
transport processes; (iv) they can serve as receptors for signal molecules; (v)
they can serve as the basis for constructing even more complex supramolecular
structures. These structures УflashФ in the cell space like signal lights,
perform their role, and disappear, to appear in another place and at another
time. The meaning of the existence of the structural УflashesФ is that during
transition into the active state the cell needs new resources, functions,
mechanisms, regulators, and signals. As soon as the cell changes to the resting
state, the need for these structures disappears, and they are disassembled.
Extreme examples of native aggregation are muscle contraction, condensation of
chromosomes, the appearance of the division spindle, and interactions of ligands
with receptors.
Thus, the present paper will consider the meaning and significance of native
aggregation as the universal structural basis of the active cell. The basis of
pathological states is the inability of the cell to return to the resting state
and errors in the formation of signal structures. The presentation of native
aggregation is based on three pillars: (i) reversible protein aggregation is a
structural basis of cell activity (Nasonov's School); (ii) the operation of the
living cell or its individual structures can be regarded as a repetitive
sequence of transitions between two states (active and resting), a key role in
which belongs to natively unfolded proteins (Ling's approach); (iii) the
specificity of interactions of separate parts of a single polypeptide chain with
each other (folding) or the interaction of separate polypeptide chains among
themselves (self-assembly, aggregation) can be provided only by protein
secondary structures.
The goal of this paper is the enunciation of principles, rather than a review of
facts corresponding to these principles.
Native aggregation in retrospective. The best-studied
nonspecific response of cells to external actions might possibly be the response
to fixatives. For a long time in the history of science, cells were considered
optically empty structures by researchers. The appearance of methods of fixation
and staining wrought a revolution in cytology, as these approaches opened to the
researchersТ sight numerous cell structures whose existence had not even been
suspected. After a period of euphoria, doubts were cast: were these structures
real or were they the results of fixation, denaturation of the cellТs
native substance?
The danger of serious errors when artifacts of fixation might be considered real
structures became a subject of general attention after 1899 (see [9], Ch. 1 for
details), when coagulation of homogenous protein solutions was shown to lead to
the appearance of structures quite similar to those observed in fixed cell
preparations (see [10], Fig. twenty-four). The shape of such artificial
structures depended on the chemical nature of the fixative, its concentration,
the protein concentration in solution, the temperature, and other conditions.
This brought about an obvious crisis in the study of cell morphology.
However, other things were also obvious. In the optically empty part of the
cell, visible structures could appear not only during fixation, but also during
the transition of the cell to the active state. Comparative observations on
fixed preparations and living cells showed that where the structure appeared in
vivo, it was also observed in a fixed preparation. The obvious resemblance
between native structures and the structures obtained as a result of fixation
gave grounds for considering that several cell structures are formed not only at
fixation, but also during activation of some particular cell fraction, when new
structures absent in the resting cell are formed by self-assembly (see [9], Ch.
1 for details).
This discussion has led to the rather important conclusion that despite the
dangers of producing artifacts, another thing is beyond doubt: in the process of
aggregation, the denatured cell proteins interact with each other not
chaotically, but regularly, in accordance with a certain plan (this is what I
call native aggregation). The laws of this interaction lead to the formation of
temporary structures necessary for the cell to function under new conditions.
During fixation and dehydration, this process initially occurs Уas it shouldФ
(the self-assembly of real cellular structures takes place), but it goes too far
when the process of making the preparation is completed, when aggregation
becomes irreversible and the structure appearing as a result of aggregation
becomes a УcorpseФ. If the interaction of proteins during aggregation had been
chaotic, we would still know little about cell structure.
The course of native aggregation seems to be determined by the non-homogeneity
of the content of the resting cell; it has structure that is invisible under the
light microscope, but reveals itself at the onset of native aggregation. The
role of structure guiding native aggregation may be played, for instance, by
PorterТs Уmicrotrabecular latticeФ [11], which can be envisaged лЕas that which
is in the background of all the visible membranous organelles and all the
visible elements of the cytoskeleton; e.g., that which has been invisible up
until now and which we wish to УseeФ microscopically╗ [12]. Such a lattice might
act as the center of УcrystallizationФ or the center of УattachmentФ of
aggregating proteins. However, this is merely an example that I cite for
clarity. The centers of crystallization can also comprise the most sensitive
proteins that are the first to respond by conformational alterations to changes
in the medium and become aggregation-competent. In any event, as a result of
native aggregation, the hidden structures become visible under the microscope.
Fulton [13], a convinced Porter devotee, moved even further: she put forward a
point of view that Уthe cytoplasm is so compact that it is only occasionally
more open than a crystalФ. Sufficient data have probably accumulated in the
literature to establish that the content of a cell is to be considered a
structured system that guides native aggregation into the required course. As
one example, one can indicate the data of Baló-Banga et al. [14]: the
birefringence of lymphocyte nuclei was enhanced after fixation with ethanol,
i.e., correct fixation leads to the appearance of new, more ordered structures.
However, especially interesting are the cases when native aggregation, as I call
it, takes place in the process of normal cell functioning. Thus, in the same
work by Baló-Banga et al. [14], activation of lymphocytes by specific antigens
or haptens was shown to lead to a significant enhancement of nuclear
birefringence. The same phenomenon was also observed in the case of activation
of peripheral blood lymphocytes with allergens in drug-allergic patients [15].
If the factor affecting the cell becomes more intense, its activating effect
will be replaced by a damaging one. Thus, the studies of Inners and Bendet [16]
on thermal DNA denaturation in bacteriophage T2 and spermatozoa [17] showed that
during irreversible denaturation of structures their capacity for birefringence
is lost. Such data indicate that under certain conditions, the actions of heat,
organic solvents, etc. on cells produce not native aggregation, but destruction,
disorganization of intracellular structure; in other words, destruction of
structure can follow native aggregation. Unfortunately, there is a marked
tendency in the literature towards rough alterations in the structure of the
cytoplasm and organelles, because they are easier to study.
Thus, the retrospective considered shows that when adequate methods of study are
used, native (programmed) protein aggregation leading to self-assembly of
various cell structures is the usual phenomenon of cell life. An example of this
is the universal reaction of the living cell [8].
Universal reaction of the living cell and native aggregation.
Why does native aggregation not occur in cells in the resting state but begins
only on activation (for instance, muscle contraction, action potential) or
damage? To answer this question, let us return to NasonovТs denaturation theory
[1]. According to this theory, excitation of the cell takes place only when its
proteins are subjected to the initial stages of denaturation.
Mirsky seems to have been the first to pay attention to the similarity between
changes in active cytoplasm and the denaturation of isolated proteins [18].
Mirsky came to the conclusion that denaturational protein changes appear when an
egg cell is fertilized [19] and during photoreception [20]. This is what he says
about it in the latter of the above-cited works: "...There is evidence
indicating that light denatures a conjugated protein, visual purple, and that
denaturation reverses in the dark." However, his studies in this direction were
not systematic.
Nasonov and his followers studied the effects of quite different factors
(chemical substances, pH, hydrostatic pressure, mechanical action) on cells of
different types. As a result, a regularity was revealed: regardless of the
character of the action and the type of cell, the response reaction represented
a monotypic (nonspecific) complex of synchronous changes. These changes were of
two-phase character: macroscopic viscosity first decreased, then rose; binding
of vital dyes by cell structures (under conditions of diffuse equilibrium) first
decreased, then increased; in the first phase of the reaction the cytoplasm
became clear, in the second phase it became turbid. Other parameters (see [8]
for review) were also studied. The first phase of this reaction is not related
to the subject of the present paper, as it is a variation of the resting state.
Of interest to us is the second phase, whose structural basis is protein
aggregation (Fig. 1). It is this phase that is the phase of activation of cell
functions [1].
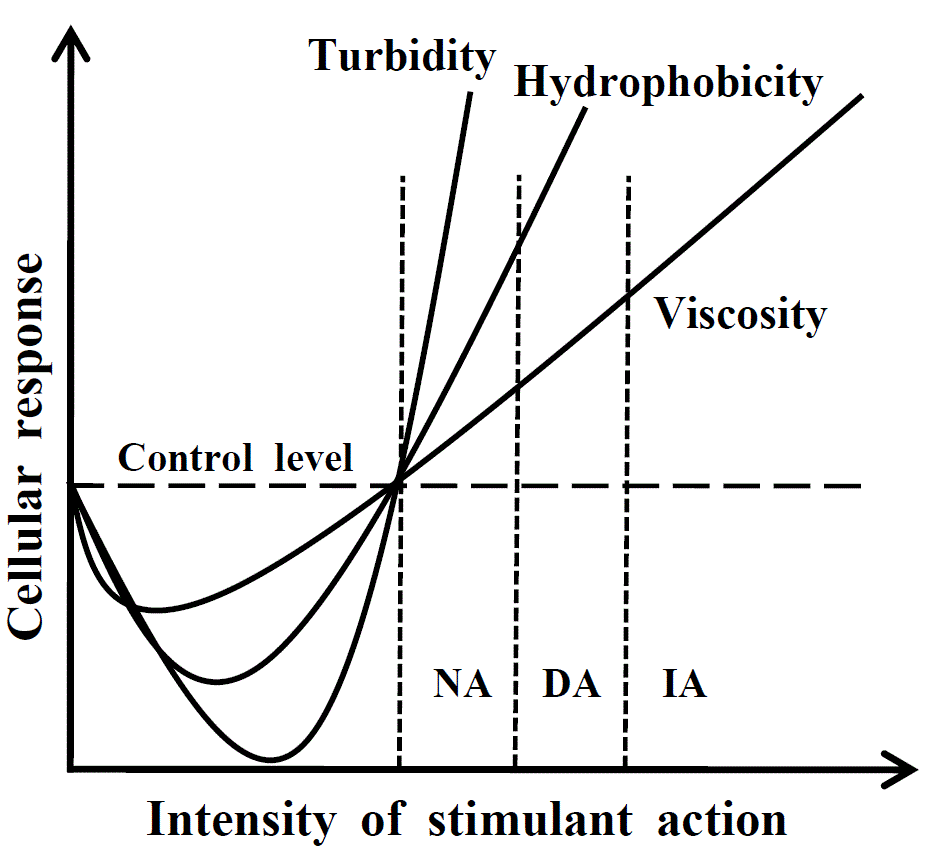
Fig. 1. Response reaction of cell depending on strength of external action
(scheme). On reaching the threshold, the first phase of the cellular response
begins; during this phase the cell becomes more transparent, while
hydrophobicity and macroscopic viscosity decrease. Then the second phase of the
cellular response begins, during which all parameters measured significantly
exceed the control level (in this case, the control level means the cell resting
state).
NA, native aggregation when necessary cellular functions and signaling pathways
are activated; DA, damaging aggregation when signals for apoptosis, cancer
transformation or other pathological cellular states may be generated; IA,
irreversible aggregation leading to cell death. See [8] for details.
This second phase was called the phase of excitation and damage by NasonovТs
school. Substantial changes in the cell in this phase are remarkably reminiscent
of denaturation of isolated proteins; therefore, Nasonov called his theory
explaining the cell response reaction the denaturational theory of excitation
and damage. According to this theory, the initial stages of denaturational
changes, when they still are reversible, underlie cell excitation (activation of
secretory function, muscle contraction, action potential, etc.). More profound
protein changes lead to disturbances of normal cell functioning, but may still
be reversible. Then, with further development of damage, denaturational changes
become irreversible and the cell dies.
The peculiarity of the cellular reaction discovered and studied by NasonovТs
school was its nonspecific character: whatever the action on the cell was, its
proteins were aggregated (as in fixation); any cellular activity was also
accompanied by protein aggregation (this is especially well seen in the case of
muscle contraction). The behavior of isolated proteins during denaturation was
the same: any denaturing agent caused their aggregation (except for denaturation
under non-physiological conditions, e.g. denaturation by concentrated solutions
of urea).
In this universalism of the cellular response, a puzzle was hidden, but in an
era concerned with specific interactions, nonspecific phenomena drew no
attention. Nevertheless, it is obvious that the nonspecific cellular reaction
discovered by NasonovТs school is a fundamental natural phenomenon Ц like cell
division or carcinogenesis. Attention to it is justified because the phenomena
of nature, unlike theories, cannot be erroneous.
The nonspecific character of the cellular reaction considered is a superficial
impression. Death is also a nonspecific phenomenon, but the processes leading to
it are characterized by diversity and can be extremely specific. In exactly the
same way, aggregation of proteins can be based on specific interactions. If we
deny the existence of specific mechanisms in cell protein aggregation, we will
not be able to understand why cell stress initiates such processes as
proliferation, differentiation, senescence, apoptosis, necrosis, or mitotic cell
death [21]. On the other hand, it is obvious that with all the specificity of
interactions leading to protein aggregation, the cellular reaction looks
nonspecific because any aggregation, whether specific or nonspecific, ends in
the formation of protein complexes. Therefore, it is more correct to focus not
on the nonspecificity, but on the universality of the complex of structural and
functional cellular changes studied by NasonovТs school. That is why I have
proposed to name this typical cellular response a universal reaction of the
living cell or protoreaction, because there are grounds to consider it the most
ancient type of cellular reaction to external actions [8].
Thus, it is the denaturation of proteins that makes these polymers active. Their
activity arises from the fact that only denatured proteins begin to interact
with each other. This interaction seems to be specific and regular; native
aggregation results in new structures that are absent in the resting state and
have physiological meaning for the active state. In other words, denaturational
changes make proteins reaction-capable. While these changes are
reversible, the cell is able to disassemble the temporary structures formed and
to return to the initial state Ц the resting state. When damage ensues, when
protein aggregation becomes too extensive or irreversible, pathological changes
appear in the cell and can lead to its death. The threshold character of the
cellular reaction means that the resting state and the active state are
different thermodynamic states of the system, which are separated by an energy
barrier; this relates not only to the cell as a whole, but also to its
individual components [5].
Now the time has come to ask: what makes protein aggregation specific? The
answer to this question is provided by the physics of proteins. It has been
established that the correct folding of a polypeptide to a globule, like the
unique structure of the globule itself, is provided by specific interactions
between protein secondary structures [7]. Let us consider a structure
such as an
α-helix. It interacts with other secondary structures via its
surface. The surface of the
α-helix is УencrustedФ with polar (hydrophilic) and non-polar
(hydrophobic) groups. Taken individually, these groups are capable only of
nonspecific interactions, but the secondary structures confer a specific
character on these interactions. This is their biological meaning. Indeed,
depending on the amino acid composition, the topography of hydrophobic groups on
the surface of an
α-helix can vary strongly. If two
α-helices have complementary topographies of hydrophobic
amino acid residues, such secondary structures will УrecognizeФ each other and
associate to form a hydrophobic nucleus (the principle of УkeyФ-to-УlockФ
correspondence works here, too). Owing to the same topographic factor,
polar groups can form on the secondary structure surface a УlandscapeФ
complementary to the nucleic acid surface. To provide specificity of interaction
by a unique distribution of protein functional groups on the surface is the main
purpose of all protein secondary structures. The principle of structural
complementarity has a universal physical basis and is realized not only in
intraprotein interactions (in the globular proteins formed and in the process of
their folding), but also in interprotein interactions (native aggregation)
including protein-nucleic acid interactions.
When an action on a cell or cell structure exceeds the threshold, (i) formation
of secondary structures begins in natively unfolded proteins (or unfolded
regions of proteins), while (ii) secondary structures of molten globules start
to become accessible for interaction with secondary structures of other proteins
and with nucleic acids. Such secondary structures induced by the external action
are centers (sites) of native aggregation. Thus, the first event in the
activated cell is the appearance of new secondary structures able to interact
selectively with each other to form tertiary, quaternary, etc. structures.
Proteins whose secondary structures appear under such circumstances lose their
previous inertia and become reaction-capable.
The proposed approach to understanding the mechanisms of cellular reactions
poses the question of native and denatured protein states in a new way. In the
native state the key cell proteins are inert, non-reaction-capable; they do not
interact with each other or with other biopolymers. Loss of the state of inertia
is denaturation. On denaturation of the unfolded polypeptide chains the
secondary structures appear, whereas on denaturation of molten globules their
secondary structures are modified and Уfloat upФ to the surface from the
hydrophobic nucleus. In both cases the secondary structures are ready to
interact. In other words, two extreme protein states can be identified: the
completely folded (the globular protein) and the completely unfolded states.
Between these inactive (native) states, numerous intermediate, active forms can
exist; it is these forms that provide native aggregation. Thus, in proteins,
only two states are inactive (they are native states). In all other cases they
are active, as manifest in the capacity for native aggregation.
The proposed mechanism of native aggregation explains the increase of volume of
the cellular hydrophobic phase during the protoreaction [8] and the structural
changes in the universal reaction of the living cell [1]. When secondary
structures form, the polar groups of peptide bonds break contact with water and
form hydrogen bonds with each other. For this reason alone the hydrophobicity of
a polypeptide with secondary structures is higher than in the unfolded
polypeptide-precursor. The volume of the hydrophobic phase increases even more
when the secondary structures fuse to form hydrophobic domains (nuclei). The
second reason why the volume of the cell hydrophobic phase increases further is
the appearance of molten globules. In native globular proteins the hydrophobic
nucleus is a solid body with a comparatively small surface interacting weakly
with hydrophobic substances (therefore, the cell in the resting state is
hydrophilic). On melting, the hydrophobic nucleus ceases to be a solid body
([7], Lecture 17); its constituent elements become much more mobile relative to
each other, and the nucleus loosens and becomes accessible to water and to
substances dissolved in it (surface hydrophobic contacts increase). If the
solution contains hydrophobic compounds, it becomes possible for them to
penetrate into the molten globule nucleus and become concentrated in this
hydrophobic phase.
Proteins in the excited state are capable not only of new intramolecular
interactions, but also of interaction with other proteins. Protein physics
offers no prohibitions on this point. Native aggregation (formation of specific
aggregates) explains the increase of cell turbidity and of macroscopic viscosity
of the cytoplasm and nucleus. Thus, the observed changes during the
protoreaction are given a simple explanation based on data from protein physics
[7].
In this section, significant attention was paid to the cell in the resting
state. Let us now consider it in greater detail.
What is the resting state of the living cell? To study any
process, it is important to identify a starting point. For instance, it would
have been impossible to understand the mechanism of muscle contraction without
the concept of the resting state of the contractile apparatus. Based on the
experience of classical physiology, it is necessary to accept that the concept
of the resting state of cell (as well as of its individual parts) is of great
importance for understanding the mechanisms of activation. Here we return again
to the issue of the structure of the resting cell. The fact that such a cell,
unlike an activated one, is almost completely transparent, indicates a
negligible amount of protein aggregate. Also, the resting cell is hydrophilic,
as under conditions of diffusional equilibrium it does not bind vital dyes [1],
which are hydrophobic [8]. These essential peculiarities of the resting cell are
to be explained by its structure.
Ling [22] was the first to suggest that the structure of the resting cell is
determined by natively unfolded proteins. This concept was finally formulated by
1965 [23], while a summary of the development of this way of thinking was
published a decade later [6]. The most important argument in favor of this point
of view is the identity of the equilibrium distribution of substances
between the cell and the medium on the one hand, and between the model systems
and the medium on the other. The model systems studied include cellophane
dialysis bags filled with concentrated solutions of hydrophilic and electrically
neutral linear polymers, all of whose chain links are accessible to
water. The distribution law, i.e., dependence of equilibrium distribution of
substances on their concentration in the medium, is the same for the model
systems and for the living cell. Since the distribution of substances was
studied under conditions of diffusional equilibrium, this result means that the
key physicochemical factor determining the character of the distribution is
identical in the models and the cell, and is provided by unfolded
biopolymers. It seems obvious that of all cell polymers, only proteins Ц the
most massive cell polymers Ц can possibly fulfill this role [23].
What is this factor? Both cells and models have a common peculiarity: if the
solution component studied is not absorbed on a polymer within the system, its
equilibrium concentration in the internal medium is always lower than in the
external solution. Model systems, owing to their simplicity, allow this
phenomenon to be understood: it is because substances are less thoroughly
dissolved in the system water than in the water of the external medium. Physics
provides the only possible explanation for this difference: water in the cell
and in the model systems is more ordered than bulk water; therefore, insertion
of a molecule of solute with more rigid bonds into the solvent is not
energetically advantageous, so solutes are displaced (excluded) from the system.
But why is water ordered in the presence of linear polymers? The obvious
explanation is provided by model systems comprising nothing but polymer, water,
and dissolved substance: if water is absorbed by the regularly repeated polymer
links, the water itself is ordered in the space (multilayer adsorption). Also,
in the absorbed water molecules, the electrical properties are different.
In spite of the wide diversity of proteins, they all have absolutely identical
polypeptide backbones; differences between proteins are due only to the side
chains. The polypeptide backbone of all proteins comprises a regular alternation
of positive (NH) and negative (CO) charges in the peptide bonds; the distance
between these groups turns out to be comparable with the size of a water
molecule and with the length of the hydrogen bonds between them. In other words,
the disposition of these dipoles along the polypeptide backbone is complementary
to water structure. Another peculiarity of the peptide bond groups is that they
form hydrogen bonds either with each other (in the secondary structures) or with
water (in the unfolded regions of the polypeptide chain) ([7], Lecture 4).
However, the question arises Ц why does the interaction of water with the
functional groups of peptide bonds change its properties so markedly? To answer
this question, let us address the properties of electric dipoles.
An important property of dipolar molecules is that their dipole moment is not
constant, but depends on their interaction with other dipoles [24]. Example: the
dipole moment of water in the gaseous phase is equal to 1.85 D, while in the
liquid phase it is 2.9 D. Hence, the interaction of water molecules with each
other leads to their mutual polarization Ц an enhancement of their own dipole
moment by 60% [25]. But what if the water molecule interacts with a stronger
dipole than itself? The dipole moment of a peptide group is 3.5 D [26]. If water
interacts with these, stronger, dipoles, its molecules will be polarized to a
greater degree and their hydrogen bonds with other molecules will become
stronger. The enhancement of hydrogen bonds makes the first adsorptional layer
stable and able to attract and to bind more and more new free water molecules,
forming more and more new adsorbed layers. Thereby, stronger dipoles on the
adsorbing surface are the key prerequisites for the multilayer adsorption of
polar molecules.
Owing to the enhancement of hydrogen bonds in the multilevel adsorbed water
layer, penetration of other molecules into it (including water itself) becomes
energetically non-advantageous, because it requires breakdown of the
intermolecular hydrogen bonds in the layer, which are stronger than in the
voluminous (bulk) phase. This explains why bound water is a poor solvent
compared with the phase in which water molecules interact only with each other.
For this thermodynamic reason, the concentration of any substance in the
absorbed phase always will be lower than in the liquid phase.
However, all begins to change if the unfolded polypeptide absorbing water begins
to fold with formation of secondary structures. In this process, peptide groups
cease to form hydrogen bonds with water and form them between each other. The
previously bound water is desorbed and acquires the properties of voluminous
(bulk) solvent [6, 23, 27]. There is convincing experimental evidence to
substantiate this point of view about the interaction of polypeptides and other
hydrophilic polymers with water [28, 29].
But what is the role of globular proteins? It is these compounds that are the
second important component of the cell in the resting state. They are the
best-studied type of proteins, performing structural and enzymatic functions.
Their solid core is inaccessible to water, while polypeptide chains containing
no secondary structures are not sufficiently expanded to affect the state of the
intracellular water fundamentally [5].
Thus, in the resting state, the physical properties of the cell protein matrix
are determined by partially or completely unfolded proteins and by globular
proteins (of course, the latter include complex proteins with several globular
domains). In the context of the present paper, such proteins can meaningfully be
called native. The structural and functional peculiarities of the cell in the
resting state are determined by unfolded proteins [5].
The question remains as to why the resting state of the cell is relatively
stable and can exist for an indefinite period. Ling believes this is accounted
for by the stabilizing effect on unfolded proteins of various ligands bound to
native unfolded proteins: ions, low-molecular organic compounds, hormones, etc.
According to Ling, the most important ligand of proteins in the resting state is
ATP [30]. If some action leads to splitting of ATP or to dissociation of other
rest-making ligands, this leads to folding of the natively unfolded protein;
secondary structures appear and make the polypeptide reaction-capable. Native
aggregation begins, in the course of which signaling structures are formed.
Natively unfolded proteins seem to be the most sensitive elements of the resting
cell, as their folded state is economically advantageous, because when the water
is desorbed the entropy of the system increases (water is the most abundant cell
component). Also, the rest-making ligands are not firmly bound to natively
unfolded proteins, as the bonds are non-covalent, while ATP can be split
enzymatically. As a result, individual cell components or the entire cell appear
as a system in which the structural content of life activity is the reversible
transition from the resting state into the activated (excitatory) state provided
by the reversible transition of proteins from the resting (native) into the
activated (non-native) state.
Principles of native aggregation. From the point of view of the
proposed approach, reactions of the cell to external actions, various forms of
cellular activity (metabolism, division, muscle contraction, secretion,
intracellular signaling, etc.) as well as pathological states are considered on
the basis of the following statements and principles.
Native aggregation is a specific interaction of proteins with each other,
realized by interaction between the secondary structures of the aggregating
proteins. If the reaction-capable secondary structures are absent or
inaccessible for interaction, native aggregation is impossible.
The cell is considered as a system that can have only two states: the resting
state and the active (excitatory) state. The same principle is true for any cell
organelle, structure or protein molecule. For clarity, a parallel can be
presented: the excitable membrane in a state of rest or excitation.
Functionally important cell proteins in the resting state are present in one of
two states: either unfolded (completely or partly Ц natively unfolded proteins)
or folded to the protein globule state or any other form in which secondary
structures are inaccessible for interaction with other proteins. These states
are considered the resting states of protein molecules or as their native
states. Proteins in the native state are stabilized by rest-making ligands
and/or chemical modifications, for instance, by phosphorylation/dephosphorylation.
According to Ling [30], the resting state of an unfolded protein is maintained
by its bound ATP, ions (Na+, K+, Ca2+),
molecules of bound water, hormones (for instance, insulin), and any other
significant interactions. For instance, analysis of amino acid sequences in the
regions surrounding known phosphorylation sites reveals a strong propensity
towards adoption of a natively unfolded conformation [31]. Disruption of bonds
with ligands (for instance, breakdown of ATP) leads to activation of the
protein, its transition from the resting to the active state; the same result is
produced by a decrease in the cell ATP content below the critical level. LingТs
concept of the capability of small molecules for specific binding with natively
unfolded proteins is confirmed, for instance, in the work by Mukhopadhyay et al.
[32].
On activation of the cell by external actions, intracellular factors, and
signals of different nature (including chemical modification), a new protein
fraction appears Ч activated proteins with newly formed secondary structures
that were absent in the resting state (Fig. 2). These new structures appear on
the folding of natively unfolded proteins and on melting of protein globules.
They include
α-helices,
β-sheets, and other secondary structure
variants. The secondary structures of activated proteins are new УvalencesФ
necessary for new interactions Ц intramolecular (folding) and intermolecular
(native aggregation). In the case of large proteins, the secondary structures
can form hydrophobic sites on the protein surface, which interact specifically
with similar (complementary) structures on the surfaces of other proteins.
The natively unfolded proteins can be called excitable proteins. Their
transition to the excitatory state triggers native aggregation.
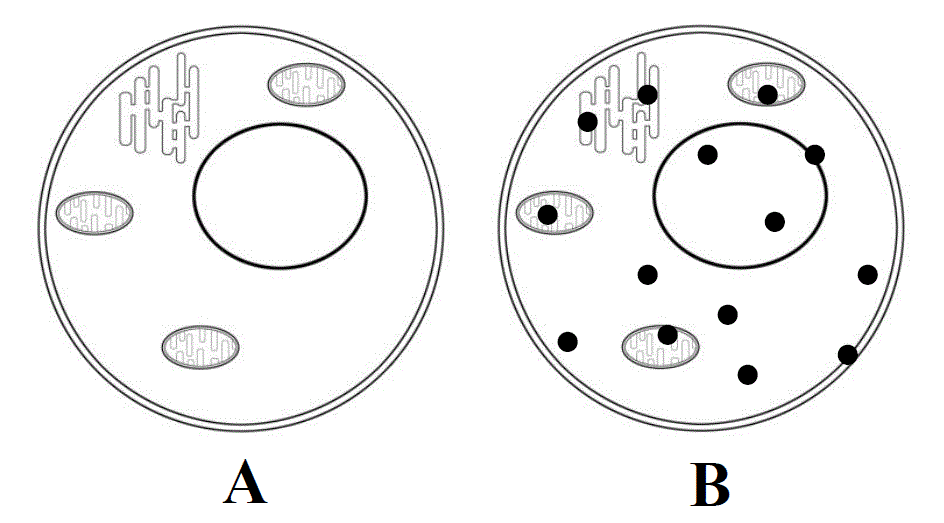
Fig. 2. Two main cell states. A - cell in the resting state, optically transparent. B - activated cell, in the cytoplasm and organelles of which native aggregation centers (closed cycles) appear - the reaction-capable secondary structures of activated proteins, owing to which native aggregation of cell proteins begins.
If on the unfolding of a globule (or a globular domain) no molten globule
intermediate is formed, while the protein cooperatively assumes the completely
unfolded configuration at once, this means it is inactivated, as a protein
without reaction-capable secondary structures is incapable of aggregation. The
molten globule may be inactivated in two ways: by transition back to the
well-folded conformation (when secondary structures are hidden from interaction)
or by unfolding of the molten globules until a completely unfolded conformation
is reached, devoid of the secondary structures that are key to native
aggregation.
The secondary structures in activated proteins play the role of centers of
native aggregation. It is these structures that provide for specific
interactions of activated proteins with each other (native aggregation) to form
new structures that have signaling and functional significance for the active
cell. Native aggregation is determined by the same forces and interactions that
are involved in the well-studied folding of unfolded polypeptides to globules.
This rule is followed: if there are secondary structures capable of specific
interaction, there is native aggregation; if there are no such structures or
they are inaccessible, there is no native aggregation.
If an action on a protein increases the number of amino acid residues included
in its secondary structures, that protein is activated and the signal pathways
in which it participates are open. If the protein is unfolded and the portion of
amino acid residues in secondary structures decreases, it undergoes transition
to the inactive state, is relaxed, while the signal pathway(s) in which it
participate(s) is/are blocked. On the transition of proteins participating in
native aggregation to the native state, native aggregates are destroyed and
individual structures and the cell as a whole transit to the resting state.
The temporary structures appearing as a result of native aggregation perform
diverse functions. They may be centers of specific adsorption (binding) of
various ions and molecules including signal factors and proteins, i.e., can
perform the functions of receptors. They may have the enzymatic activity
necessary for performing specific functions and may serve as centers of
formation of even more complex supramolecular structures.
Only the secondary protein structures are able to provide for specificity
(selectivity) in the interaction of proteins with others, as they provide the
specificity of interactions necessary for correct folding of the polypeptide
chain to a globule (the folding of polypeptide to native globule can be
considered as intramolecular native aggregation). Each secondary structure has a
unique topology of polar and hydrophobic groups on its surface. Secondary
structures form stable complexes with each other or with sites on nucleic acids
only if their surfaces are complementary to each other, as the key is
complementary to the lock.
Native aggregation is determined genetically to the same extent as protein
structure because it is determined by the same factors that determine all levels
of organization of the individual protein molecule beginning with the primary
sequence. Secondary structures of activated (excited) proteins will interact
with other excited proteins not chaotically, but in accordance with the genetic
program. As a result of native aggregation, those structures and corresponding
functions will appear that are necessary to the cell here and now: action
potential, channels on the cell surface, in the cytoplasm and nucleus,
cytoskeleton, movement of cytoplasmic sites, cell division, apoptosis. Errors in
native aggregation that appear during a prolonged state of cell excitation (for
instance, chronic inflammation) and on damage lead to various forms of cellular
pathology: conformational diseases, necrosis, carcinogenesis.
All the differences between the excited cell and the cell in the resting state
are the direct or indirect results of native protein aggregation.
Native aggregation in action. Since practically any change in
the cell can be considered a result of native aggregation, I will focus on only
a few examples. The aim of this section is to show how the principles of native
aggregation work in the analysis of particular phenomena.
I will begin with the natively unfolded proteins, the physical basis of the
resting state. According to Dunker et al. [33], the first data about natively
unfolded regions in proteins appeared in 1978, i.e., 26 years after Ling [22]
had first suggested their existence. Until the discovery of natively unfolded
proteins, the dominant notion was that the whole diversity of cell functions is
due only to proteins with 3D structure. Natively unfolded proteins were not
compatible with this notion and it was not clear whether they performed any
function at all. Subsequently it was found that more than 35-51% of eukaryotic
proteins had unfolded regions longer than 50 consecutive amino acid residues,
which is significantly higher than in prokaryotes [34, 35].
When it became clear that natively unfolded proteins played an important role,
Dunker et al. [33] proposed to widen the notion of functional protein types in
the cell: to proteins with 3D structure, they added molten globules and proteins
with unfolded conformations. Uversky [36] proposed to supplement this list with
a fourth, relatively stable protein conformation - the premolten globule, which
might be called the boiling globule, as in the coordinates of the unfolding
reaction it follows the globule and molten globule and precedes the completely
unfolded conformation. The rationale of this proposal is that all four protein
states are thermodynamically stable, although to different degrees.
In the opinion of Dunker et al., transitions between different phasic states
continually take place in the cell. This is so, indeed; however, the statement
needs clarification. Let us recollect that the first ideas about the molten
globule and unfolded protein conformation were obtained by studying protein
denaturation in vitro and then they were extrapolated to the cell. Nowadays we
know that globule melting is a phase transition that fits the "all-or-nothing"
law and has a threshold, for instance, a temperature threshold [7]. This means
that several similar molecules under identical conditions will be in the same
phase state: either globule, or molten globule, or the unfolded conformation.
Within such a population, uninterrupted and asynchronous protein transitions
from one phase state to another cannot take place. However, molecules of the
same protein located in different microenvironments can be in different phase
states, but the state may also be identical for all proteins of the same (given)
population. As a result, we find that this (some) protein can indeed be in
different phase states in this cell, but only if its molecules are located in
different parts of the cell with different microenvironmental conditions.
Another specification is also to be made. According to the hypothesis of native
aggregation, there are only two basic protein states in the resting cell:
globules (here, proteins composed of two and more globular domains may be
included) and the natively unfolded state. Other transitional states appear in
the cell temporarily. They appear on reaching the threshold, when some factor in
the medium begins to produce a moderately (gentle) denaturing action. Then a
globular protein is melted, after which it unfolds (if the strength of the
action keeps rising), while natively unfolded proteins begin to fold. The
differences between the main states are fundamental: the globular conformation
is stabilized mainly by hydrophobic interactions, the natively unfolded one by
ATP and other ligands. As soon as the medium conditions return to normal, the
excited proteins are relaxed and the system returns to its main state - the
resting state.
Since native aggregation results in the appearance of signaling and regulatory
structures, it is obvious that as biological organization becomes more
complicated during evolution, more and more novel mechanisms of regulation of
the active cell are needed. This need is realized with the aid of new natively
unfolded proteins and, accordingly, of new transitory conformations appearing as
they fold.
In the literature, the mechanism of interaction of natively unfolded proteins
with protein targets has been widely discussed. Most commonly, four stages of
such interaction are identified: (i) random collision of natively unfolded
protein with target; (ii) weak, nonspecific interaction of natively unfolded
protein with target; (iii) formation of secondary structures in natively
unfolded protein; (iv) owing to these nascent secondary structures, a firm
complex of the natively unfolded protein with the protein-target is formed [37,
38].
In terms of the hypothesis of native aggregation, this scheme looks
unconvincing. Indeed, it is hard to imagine a mechanism (for instance, the
mechanism of muscle contraction) or a process in the living cell working on the
basis of random collisions. First, if natively unfolded proteins and their
targets collide randomly, it means that they are diffusing freely in the
cytoplasm or nucleus, i.e., we are dealing with a Brownian mechanism of
regulation. Second, if the first stage of interaction of the natively unfolded
protein with the target is accepted as nonspecific, this will mean that the
number of interactions of the diffusing natively unfolded protein will greatly
exceed the number of interactions necessary for the act of regulation. Under
such conditions, the correct regulatory response looks more random than regular.
From the point of view of native aggregation, these events appear differently.
The available experimental data indicate that natively unfolded proteins are
organized in clusters and oriented in space mainly in parallel to each other
([5], Ch. 11), while the protein concentration in the cytoplasm reaches 200-400
mg/mL [39]. Thus, under conditions of crowding, when the space between protein
molecules is not large and is filled with bound water ([5], Ch. 11), it is
difficult to imagine diffusion of free proteins. According to Ling, the protein
matrix of the resting cell is not chaotic, but structured. In terms of the
hypothesis of native aggregation this means that the program of protein-protein
interactions is responsible for the spatial distribution of the key matrix
elements (for instance in the contractile apparatus). Natively unfolded protein
does not diffuse in anticipation of a random hit to the target. The target is
relatively immobile and is located nearby. In the resting state they do not
interact with each other, as they are in the inactive (native) state, i.e., do
not have reaction-capable secondary structures.
If secondary structures are formed in the natively unfolded proteins during any
collision with other proteins, this will also become a random event and the
interactions of secondary structures with each other will not be amenable to any
logic. For this reason, random, nonspecific interactions are to be eliminated
from the mechanism of functioning of natively unfolded proteins. To prevent
random interactions from causing excitation of the natively unfolded state, such
proteins must be sufficiently stable. According to the proposed approach, the
natively unfolded proteins are stabilized by various ligands depending on their
property, location, and function [6].
The fourth stage of interaction with the target is also problematic because the
activated native protein will interact, in my opinion, only with activated
protein-target (with its active secondary structures). Native globular proteins
(or globular domains in large proteins) in the native state do not have
secondary structures accessible for external interactions. This is prevented by
the rigid nuclear structure of such proteins ([7], Lecture 13).
Thus, we see that the hypothesis of native aggregation differs from the model
accepted in the literature in that it involves nothing random and nonspecific.
Moreover, it contains elements of control and management: genetic control of the
primary sequence (hence, also the properties of secondary structures), ligands,
highly specific interactions of secondary structures with each other, and
spatial control of the course of native aggregation.
As for spatial control, it is also provided first of all by interactions of
"residual" secondary structures of neighboring natively unfolded proteins (from
the point of view of the proposed approach). This is quite a substantive
suggestion, if we take into account that the complete absence of secondary
structures is possible under the most severe conditions ([7], Lecture 17). If we
also take into account the selective binding role of "residual" secondary
structures, the spatial structure of the protein matrix in the resting state is also under genetic
control, as properties of the "residual" secondary structures are encoded by the
primary sequence of amino acid residues.
Now let us consider the properties of a molten globule in greater detail.
Packing of
polypeptide chain of normal globule is so dense that the side chains are tightly
apposed to each other and their rotation around valence bonds (turn isomerization) is impossible. When the nucleus melts, the globules increase in
volume by approximately 50% [36]; free volume appears and, concomitantly, turn
isomerization also becomes possible. As a result of nuclear loosening, water and
hydrophobic substances (for instance, the dye ANS) begin to penetrate into the
nucleus. If the intensity of the denaturing factor rises, the molten globule is
converted into a premolten globule, in which the amount of secondary structure
is approximately half that in the molten globule ([7], Lecture 18).
These properties of the molten globule (to say nothing about the premolten one)
indicate that its nucleus loses rigidity and more closely resembles a fluid. An
elevation of conformational temperature inevitably leads to increased mobility
of parts of the molecule and to a decrease of the portion of the polypeptide
chain included in secondary structures. Modification of secondary structures
inevitably leads to a change of their specificity due to a change of their
topological characteristics. In other words, a change in size of secondary
structure (for instance, length of
α-helix) means a change in the biological meaning of the
polypeptide "sentence". The logic of this statement has been confirmed
experimentally in studies indicating that the nucleus of a molten globule is
structurally labile [40]. Thus, the molten globule is converted into a
reaction-capable protein that can participate in native aggregation.
Next, let us consider data indicating the involvement of the protein secondary
structures in mechanisms of signal transmission. Kim et al. [41] studied the
dynamics of the cytoplasmic domains of the E. coli chemotaxis receptor on
interaction with repellent and attractant. These authors concluded that an
attractant decreases the number of secondary structures in the domain, which
blocks signal transmission into the cytoplasm. A repellent produces the opposite
effect: it increases the amount of secondary structures in the domain, and this
makes the signal function of the receptor possible. In terms of the hypothesis
of native aggregation, repellent converts the domain into the excited state,
when its "valence" for interactions necessary for signal transmission appears.
The authors also believe that methylation/demethylation of receptors is so
important for their clustering and the dissociation of the formed clusters
because it causes significant changes in the amount of secondary structures in
domains.
Williams et al. [42] note that the orderliness of a polypeptide chain is closely
connected with protein function. Thus, for instance, binding of ligands to
streptavidin, purine nucleoside phosphorylase, hypoxanthine-guanine
phosphoribosyl transferase, hemoglobin, and myoglobin leads to some
disorderliness in the protein molecules. The authors performed thermodynamic
analyses of the actions of agonists and antagonists on the corresponding
receptors and came to the conclusion that mechanism of action of these ligands
was connected to opposite effects on the orderliness of the receptor structure;
denser polypeptide chain packing inside the protein leads to enhancement of the
degree of receptor oligomerization, while less dense packing decreases the
degree of oligomerization. Interestingly, agonists produce opposite structural
changes in different receptors. Thus, while an agonist of receptor 1 increases
polypeptide chain packing in receptor 1, an agonist for receptor 2 decreases the
packing in receptor 2. The same principle applies to antagonists. The
physiological sense of these changes will be understood only when it becomes
clear which part of which signaling pathway these changes constitute. Receptors
are one more system for which the existence of two states - resting and
activated - seems obvious. In this sense, the cell may be considered a
megareceptor: conversion from one state into another produces a complex signal
to neighboring cells.
According to current concepts, chaperones play an important role in cell life.
An example of interest is the small heat-shock proteins, a variable class of
chaperones widely distributed in cells of various types. Some representatives of
this family are inactive in cells in the resting state and are activated, for
instance, on heating [43]. According to the logic of native aggregation, the
triggering action of heat not only leads to the appearance of non-native protein
forms, but also activates the heat-shock proteins themselves. For this, they
must necessarily have either natively unfolded polypeptide chain regions or the
ability to be converted into the molten globule state. This will lead to
formation of a native aggregation center and then to aggregation itself. Native
aggregation of an activated chaperone with an activated target
begins.
The presence of natively unfolded regions in chaperone molecules has been
accepted in the literature as necessary for their work [44-46]. From the point
of view of native aggregation, these unfolded sites are needed for the formation
of the secondary structures necessary for native aggregation with a target. But
the target itself is an excited protein that can become either a natively
unfolded protein (or have natively unfolded protein regions) or a globule that
becomes a molten globule. This suggestion is confirmed by the studies of Hegyi
and Tompa [47], who showed that natively unfolded proteins have no tendency to
interact with chaperones. This result is understood. Natively unfolded proteins
are proteins in the resting state. To interact with other proteins, including
chaperones, they must be activated - to be converted into the excited, denatured
state. On the other hand, chaperones have long been known to be able to interact
with molten globules [48].
From the point of view of the proposed approach, the results of native
aggregation are new structures necessary for the excited cell. The formation of
such structures is a cooperative process that needs the participation of two or
more proteins. Without such cooperation, the new structure cannot be created.
With such an understanding of native aggregation, it becomes obvious that each
of the two or more proteins, when interacting with each other, helps the correct
folding of the protein-partner. In other words, all proteins participating in
native aggregation are chaperones for each other, but some of them might be more
profoundly specialized in this direction.
It is well known that for the release of protein-targets, some chaperones need
ATP [43]. This fact is well explained in terms of Ling's concept: binding of ATP
leads to disassembly of the secondary structures formed in the natively unfolded
regions of the chaperone molecules bound to the protein-target. As a result, the
complex of chaperone with target is split. For other chaperones, the role of ATP
can be played by different ligands.
Native aggregation, like any other process in the cell, can be an object of
regulation. Its course can be affected by various factors that produce new
signaling structures. As an example, programmed cell death can be considered.
The mechanism of genetic regulation of apoptosis can be a source of the signal
that leads, as a result of native aggregation, to the appearance of a structure
that will trigger the whole cascade of reactions necessary for cell degradation.
From the point of view of native aggregation, such a structure can appear in any
part of cell - in the nucleus, cytoplasm, organelles, or plasma membrane. The
"structure of death" produced by native aggregation can also appear if the cell
(or any of its parts) is damaged. By the same mechanism, other cell pathologies,
for instance cancer, can appear.
With reference to the peculiarities of cancer cells, I would like to note one
feature that is directly related to the subject of the present paper. The
content of bound water in cancer cells is known to be lower than in precursor
cells [49]. It is on the basis of this difference that the technology of
magnetic resonance imaging allows malignant tumors to be recognized
non-invasively. From Ling's point of view, this means there are fewer natively
unfolded proteins in the cancer cell than in the normal cell. At the same time,
it has been shown in silico that the natively unfolded regions are more
extensive in cancer cell proteins: in cancer-associated proteins, the number of
such areas is 70% greater, while in signaling proteins it is 5 times greater
[50]. It is obvious that natively unfolded proteins represent a diverse
population and are directly involved in cell transformations of pathological
character.
Dynamics of the hydrophobic phase of the living cell. As I
already mentioned, the cell in the resting state is a hydrophilic system. This
is confirmed by data on the distribution of hydrophobic substances (vital dyes)
between cell and medium under conditions of steady-state distribution:
the cell in the resting state does not adsorb such substances [1].
Here I would like to draw the reader's attention to a very important
circumstance: under conditions of diffusional equilibrium, the plasma membrane
stops working as a barrier to a diffusing substance. There are no absolutely
impermeable membranes, especially for hydrophobic substances. The dye
undoubtedly penetrates into the resting cell, but is not accumulated in it. Why?
There are two reasons: in the cell, hydrophobic binding centers for dyes are
absent; and intracellular water is a poor solvent for them. For these reasons,
the dye molecules penetrating into the cell are eventually pushed out into the
medium. Thereby, under conditions of steady-state distribution, the
character of the distribution of the substance between cell and medium is
determined by only two factors: sorption on intracellular structures, and the
low solving capability of intracellular water ([51], Ch. 5).
Everything changes when the cell is converted to the excited state: binding of
vital dyes under conditions of steady-state distribution rises by tens or
hundreds percent of times [1]. Only one explanation for this is possible: the
volume of the hydrophobic phase in the cell increases explosively [8].
The hydrophobic phase is habitually associated with the membrane lipid phase;
but the volume of the lipid phase is negligible compared with cell size and,
what is most important, it cannot rise tens of times in fractions of second.
However, as we already know, proteins in the excited cell undergo denaturational
changes [1]. Hence, the cause of the increase of cell hydrophobicity should be
looked for in proteins rather than in lipids [8].
The hypothesis of native aggregation provides a simple explanation for the
hydrophobic burst in the cell: the cause of the increase of the hydrophobic
phase is the appearance of excited proteins. Indeed, according to the proposed
approach, when the threshold of perturbating action on natively unfolded
proteins is exceeded, secondary structures begin to form. These structures, in
the course of native aggregation, are then included in the hydrophobic areas of
new structures - structures of excitation. As stated earlier, the hydrophobic
areas are formed not only by molten globules, but also by the secondary
structures appearing on the folding of unfolded protein regions.
The high rate of formation of secondary structures, within the microsecond time
range ([7], Lecture 9), also determines the high rate of native aggregation
overall, which explains the hydrophobic burst in the excited cell. On the
reverse transition to the resting state, the cell again becomes hydrophilic.
According to the hypothesis of native aggregation, significant changes in the
hydrophobic phase can take place in any cellular structure, including membranes
and organelles.
The proposed existence of temporary hydrophobic protein phases explains
interesting phenomena known from pharmacology, when the efficiency of a
therapeutic agent depends on the degree of functional activity of the target
cell. The best known example of this seems to be verapamil. This hydrophobic
compound [52] scarcely affects the normal heart rhythm, but very efficiently
inhibits tachycardia. The same regularity is also observed in the action of
verapamil on skeletal muscle. This dependence can be explained if, on
excitation, verapamil-binding hydrophobic receptors appear in the muscle fiber.
The effect of verapamil is due to its blocking action on slow calcium channels;
but from the point of view of the principles of native aggregation, the cell can
also contain other dynamic hydrophobic targets for pharmacological agents of
various types. In other words, using the native aggregation principles, it is
possible to predict the existence of drugs acting only on the active cell; their
targets can be located not only in the membrane (as in the case of verapamil),
but also in other parts of the cell. Such medications will produce no marked
effect on cells in the resting state (the healthy state).
The role of the dynamic hydrophobic protein phase in the life of the cell has
not been studied at all. It is unknown in the equations of cell physiology. At
present, one can discuss the significance of this X-factor only in terms of very
general regularities based on simple physical principles. For instance, it is
obvious that the appearance of the hydrophobic phase in a cell will cause the
redistribution of all hydrophobic compounds including ATP [8]. The
redistribution of hydrophobic substances between the cell and the medium will
also begin.
However, the redistribution of substances is triggered not only by the
appearance of the temporary hydrophobic phase, but also by the desorption of
water from protein surfaces. As secondary structures start to form, the adsorbed
water will become free and the "bad" solvent will become "good". This will lead
to a rapid invasion of small solute molecules into the areas that were
previously occupied by adsorbed water. If we take into account the rapid rate of
formation of secondary structures ([7], Lecture 9), it becomes obvious that
during the fast destruction of the ordered water structure, sharp concentration
gradients of such substances will appear. In the case of ions, everywhere in the
cell, in microvolumes, significant diffusional potentials will appear that may
prove to be one cause of the appearance of molten globules. Significant
concentration gradients of dissolved substances can also appear when the ordered
water layers are restored, as the rate of their restoration will also be
determined by the high rate of disassembly of secondary structures in activated
proteins.
It is obvious that during the course of native aggregation the density and
rigidity of the protein matrix will increase owing to a rise in the number of
interprotein contacts. This provides even more difficulties for models of cell
function regulation that base their mechanisms on the free diffusion of
substances in the cell, since with an increase of protein matrix density the
significance of diffusional processes will decrease.
If we return to the cell protoreaction, it can be concluded with certainty that
the hypothesis of native aggregation has managed to explain the rise of
viscosity and turbidity of the cytoplasm (Fig. 1) as well as the increase of
volume of the cell hydrophobic phase. From the proposed mechanism it is clear
that the changes discussed will occur synchronously, as the key link among all
these changes is the structural readjustment of the same key proteins.
Conclusion. The cornerstone of the hypothesis of native aggregation is the generation in proteins of temporary secondary structures that can interact selectively with secondary structures in the same or other proteins. The nonspecific reaction of cells, which was studied by Nasonov's school, turns out in reality to comprise myriads of specific protein-protein interactions. Since native aggregation is directed by active secondary protein structures, it proves to be completely under genetic control, so the dogma of Anfinsen [53] formulated for the folded polypeptide chain can be extended by incorporating native aggregation into its sphere of application.
Acknowledgments. I am very grateful to Paul Agutter, James Clegg, Ilya Digel, Laurent Jaeken, José Neira and Richard Wiggins for valuable critical comments on this article. I also appreciate Leonid Pevzner's assistance in preparation of this paper.
References
1. Nasonov DN:
Local Reaction of Protoplasm and Gradual Excitation (English
Transl. by Halpern, Y.S.), Washington, D.C.: National Science Foundation,
available at Office of Technical Services, US. Department of Commerce; 1962.
2. Ling GN:
A Physical Theory of the Living State: The Association-Induction
Hypothesis. Waltham MA: Blaisdell; 1962.
3. Ling GN:
In Search of the Physical Basis of Life. New York: Plenum Publ Corp;
1984.
4. Ling GN:
A Revolution in the Physiology of the Living Cell. Malabar FL: Krieger Publ
Co; 1992.
5. Ling GN:
Life at
the Cell and Below-Cell Level: the Hidden History of a Fundamental Revolution in
Biology. New York: Pacific Press: 2001. (The
Russian translation was published in 2008).
6. Ling GN:
A convergence of experimental and theoretical breakthroughs affirms
the PM theory of dynamically structured cell water at the theory's 40th
birthday. In Water and the Cell. Edited by Pollack GH, Cameron IL and Wheatley
DN. Berlin, New York: Springer Verlag; 2006:1-52.
7. Finkelshtein AV, Ptitsyn OB: Physics of Proteins. Amsterdam: Academic Press;
2002.
8. Matveev VV:
Protoreaction of protoplasm. Cell Mol Biol 2005, 51:715-723.
9. Wilson, EB: The Cell in Development and Heredity. New York: Macmillan; 1928.
10. Porter KR:
The cytomatrix: a short history of its study. J Cell Biol 1984,
99:3s-12s.
11. Porter KR, Tucker JB: The ground substance of the living cell. Sci Am 1981,
244:56-67.
12. Heuser J: Whatever happened to the 'microtrabecular concept'? Biol Cell
2002, 94:561-596.
13. Fulton AB: How crowded is the cytoplasm? Cell 1982, 30:345-347.
14. Baló-Banga JM, Molnár L, Nováki M, Leibinger J:
Measurement of lymphocyte
activation by a chromatin topo-optical reaction. Mechanism and specificity of
the test. Arch Dermatol Res 1980, 269:239-251.
15. Baló-Banga JM, Molnár L, Nováki M, Leibinger J: Measurement of lymphocyte
activation by a chromatin topooptical reaction. II. Application for detecting
drug allergy. A clinical and experimental study. Allerg Immunol (Leipz) 1980,
26:137-153.
16. Inners D, Bendet IJ: Thermal stability of T2 DNA in situ. Virology 1969,
38:269-277.
17. Bearden J Jr, Bendet IJ: Birefringence of spermatozoa. I. Birefringence
melting of squid, bull, and human sperm nucleoprotein. J Cell Biol 1972,
55:489-500.
18. Mirsky AE, Pauling L: On the Structure of Native, Denatured, and Coagulated
Proteins. Proc Natl Acad Sci USA 1936, 22:439-447.
19. Mirsky AE: Protein coagulation as a result of fertilization. Science 1936,
84:333-334.
20. Mirsky AE:
The Visual Cycle and Protein Denaturation. Proc Natl Acad Sci USA
1936, 22:147-149.
21. Proskuryakov SY, Konoplyannikov AG, Gabai VL:
Necrosis: a specific form of
programmed cell death? Exp Cell Res 2003, 283:1-16.
22. Ling GN: The role of phosphate in the maintenance of the resting potential
and selective ionic accumulation in frog muscle cells. In Phosphorus metabolism
vol. II. Edited by McElroy WD and Glass B. Baltimore: The Johns Hopkins
University Press; 1952:748-795.
23. Ling GN:
The physical state of water in living cell and model systems. Ann
NY Acad Sci 1965, 125:401-417.
24. Spackman MA, Munshi P, Dittrich B:
Dipole moment enhancement in molecular
crystals from X-ray diffraction data. Chemphyschem 2007, 8:2051-2063.
25. Kemp DD, Gordon MS:
An interpretation of the enhancement of the water dipole moment due to the
presence of other water molecules. J Phys Chem A 2008, 112:4885-4894.
26. Collins JM, Leadbeater NE:
Microwave energy: a versatile tool for the
biosciences. Org Biomol Chem 2007, 5:1141-1150.
27. Ling GN:
Nano-protoplasm:
the ultimate unit of life. Physiol Chem Phys Med NMR 2007, 39:111-234.
See
also:
Ling GN, Ochsenfeld MM.
A historically significant study that at once disproves the membrane (pump)
theory and confirms that nano-protoplasm is the ultimate physical basis of
life--yet so simple and low-cost that it could easily be repeated in many high
school biology classrooms worldwide. Physiol Chem Phys Med NMR.
2008;40:89-113.
28. Zheng JM and Pollack GH:
Long-range forces extending from polymer-gel
surfaces. Phys Rev E 2003, 68:031408.
29. Zheng JM, Chin WC, Khijniak E, Khijniak E Jr, Pollack GH:
Surfaces and
interfacial water: evidence that hydrophilic surfaces have long-range impact.
Adv Colloid Interface Sci 2006, 127:19-27.
30. Ling GN: The physical state of water and ions in living cells and a new
theory of the energization of biological work performance by ATP. Mol Cell
Biochem 1977, 15:159-172.
31. Iakoucheva LM, Radivojac P, Brown CJ, O'Connor TR, Sikes JG, Obradovic Z,
Dunker AK: The importance of intrinsic disorder for protein phosphorylation.
Nucleic Acids Res 2004, 32:1037-1049.
32. Mukhopadhyay S, Krishnan R, Lemke EA, Lindquist S, Deniz AA: A natively
unfolded yeast prion monomer adopts an ensemble of collapsed and rapidly
fluctuating structures. Proc Natl Acad Sci USA 2007, 104:2649-2654.
33. Dunker AK, Lawson JD, Brown CJ, Williams RM, Romero P, Oh JS, Oldfield CJ,
Campen AM, Ratliff CM, Hipps KW, Ausio J, Nissen MS, Reeves R, Kang C, Kissinger
CR, Bailey RW, Griswold MD, Chiu W, Garner EC, Obradovic Z: Intrinsically
disordered protein. J Mol Graph Model 2001, 19:26-59.
34. Dunker AK, Obradovic Z, Romero P, Garner EC, Brown CJ: Intrinsic protein
disorder in complete genomes. Genome Inform Ser Workshop Genome Inform 2000,
11:161-171.
35. Dunker AK, Brown CJ, Lawson JD, Iakoucheva LM, Obradovic Z: Intrinsic
disorder and protein function. Biochemistry 2002, 41:6573-6582.
36. Uversky VN: Natively unfolded proteins: a point where biology waits for
physics. Protein Sci 2002, 11:739-756.
37. Eliezer D, Palmer AG 3rd: Proteins hunt and gather. Nature 2007,
447:920-921.
38. Wright PE, Dyson HJ:
Linking folding and binding. Curr Opin Struct Biol
2009, 19:31-38.
39. Ellis RJ: Macromolecular crowding: obvious but underappreciated. Trends
Biochem Sci 2001, 26:597-604.
40. Lala AK, Kaul P: Increased exposure of hydrophobic surface in molten globule
state of alpha-lactalbumin. Fluorescence and hydrophobic photolabeling studies.
J Biol Chem 1992, 267:19914-19918.
41. Kim SH, Wang W, Kim KK: Dynamic and clustering model of bacterial chemotaxis
receptors: structural basis for signaling and high sensitivity. Proc Natl Acad
Sci USA 2002, 99:11611-11615.
42. Williams DH, O'Brien DP, Sandercock AM, Stephens E: Order changes within
receptor systems upon ligand binding: receptor tightening/oligomerisation and
the interpretation of binding parameters. J Mol Biol 2004, 340:373-383.
43. Haslbeck M, Franzmann T, Weinfurtner D, Buchner J: Some like it hot: the
structure and function of small heat-shock proteins. Nat Struct Mol Biol 2005,
12:842-846.
44. Weikl T, Abelmann K, Buchner J:
An unstructured C-terminal region of the
Hsp90 co-chaperone p23 is important for its chaperone function. J Mol Biol 1999,
293:685-691.
45. Richardson A, Schwager F, Landry SJ, Georgopoulos C: The importance of a
mobile loop in regulating chaperonin/co-chaperonin interaction: humans versus
Escherichia coli. J Biol Chem 2001, 276:4981-4987.
46. Tompa P, Csermely P:
The role of structural disorder in the function of RNA
and protein chaperones. FASEB J 2004, 18:1169-1175.
47. Hegyi H, Tompa P:
Intrinsically disordered proteins display no preference
for chaperone binding in vivo. PLoS Comput Biol 2008, 4:e1000017.
48. Rawat U, Rao M: Interactions of chaperone alpha-crystallin with the molten
globule state of xylose reductase. Implications for reconstitution of the active
enzyme. J Biol Chem 1998, 273:9415-23.
49. Chaplin M: Do we underestimate the importance of water in cell biology? Nat
Rev Mol Cell Biol 2006, 7:861-866.
50. Iakoucheva LM, Brown CJ, Lawson JD, Obradovic Z, Dunker AK: Intrinsic
disorder in cell-signaling and cancer-associated proteins. J Mol Biol 2002,
323:573-584.
51. Troshin AS:
Problems of cell permeability. London, New York: Pergamon Press,
1966.
52. Kasim NA, Whitehouse M, Ramachandran C, Bermejo M, Lennernäs H, Hussain AS,
Junginger HE, Stavchansky SA, Midha KK, Shah VP, Amidon GL: Molecular properties
of WHO essential drugs and provisional biopharmaceutical classification. Mol
Pharm 2004, 1:85-96.
53. Anfinsen CB: Principles that govern the folding of protein chains. Science
1973, 181:223-230.
Full text [PDF]
ARTICLES CITING THIS ARTICLE
2011 ─юІхэъю ╬.╚. ш ╠шїхэъю ┬.╬. ┬ыш эшх эшчъюірёҐюҐэющ тшсЁрІшш эр ъшёыюҐэґ■ ЁхчшёҐхэҐэюёҐ№ ¤ЁшҐЁюІшҐют. ┬iёэшъ ─эiяЁюяхҐЁютё№ъюую ґэiтхЁёшҐхҐґ. ┴iюыюуi . ┼ъюыюуi . ┬шя. 19, Ґ.1. ╤. 22-30.
Web-Links
The journal "Physiological Chemistry and Physics and Medical NMR"
Pollack G.H. Water, Energy and Life. (Lecture)